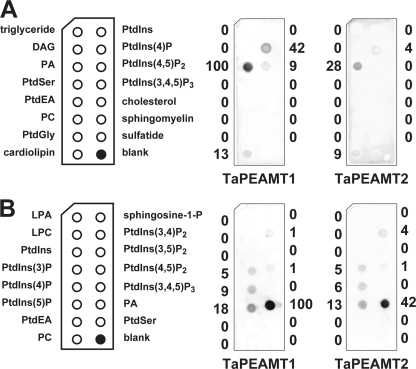
FIGURE 5.
Screen of putative lipid ligands for the two TaPEAMT isoforms. A, membrane lipid strips spotted with 100 pmol of each lipid were incubated with 80 nm recombinant TaPEAMT protein as before. As expected both isoforms strongly interact with PA, followed by some binding to PtdIns(4)p > PtdIns(4,5)P2/cardiolipin. Numbers next to each chemiluminescent signal are given as percent spot intensity of PA binding to TaPEAMT1. B, to test the specificity of the interaction with phosphoinositides PIP strips (spots contain 100 pmol of lipid each) were incubated with 80 nm recombinant TaPEAMT protein as before. The strongest interaction again is observed with PA, followed by interactions with the three PtdIns monophosphates ((5)P > (4)P > (3)P) and two of the PtdIns diphosphates ((3,4)P2 ≥ (4,5)P2) consistent with earlier findings, except that TaPEAMT2 binding to PtdIns(4,5)P2 was not detected in A most likely due to a more stringent wash. The recognition appears to be specific, because no binding to PtdIns, PtdIns(3,5)P2, or PtdIns(3,4,5)P3 was observed. Numbers next to each chemiluminescent signal describe spot intensities expressed as percentage of spot intensity for PA binding to TaPEAMT1.