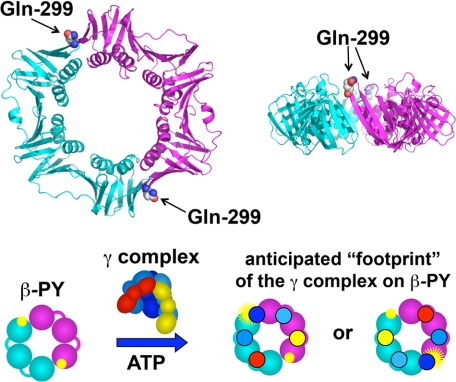
FIGURE 1.
Fluorescence intensity-based β clamp binding assay. Upper panel, ribbon diagram of the β clamp is shown with one monomer in cyan and the other in magenta. Glutamine 299 (spheres), located on the surface of β to which the γ complex binds, was converted to cysteine. Two surface cysteines, Cys-260 and Cys-333, were converted to serine, so that Cys-299 could be selectively labeled with PY. Lower panel, the β clamp has a C2 axis of symmetry through the center of the ring such that two PY fluorophores (yellow starbursts) covalently attached to Cys-299 are located on opposite sides of the ring. When the γ complex binds βPY, one γ subunit (dark blue) likely binds at or near a PY to alter its environment and increase PY fluorescence (larger yellow starburst). The second PY molecule is unlikely to interact with the γ complex so that only a single PY molecule is reporting on the binding interaction.