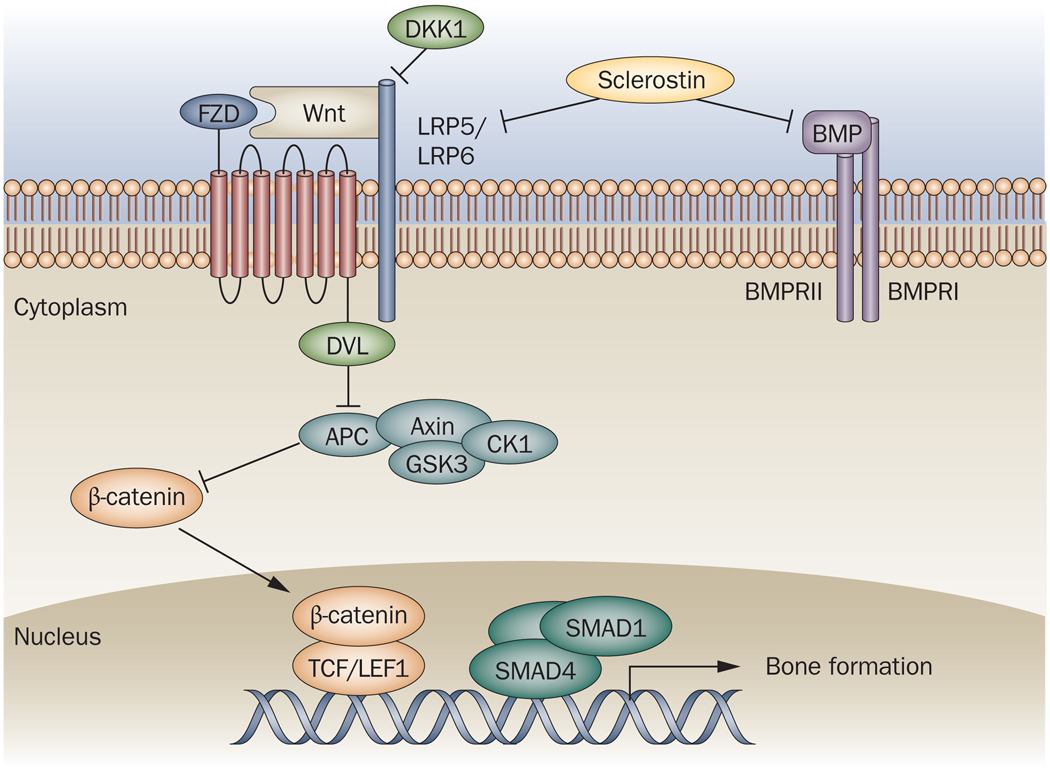
Figure 2. Osteoblast differentiation pathways.
Osteoblast formation from mesenchymal progenitors is controlled by the canonical Wnt–β catenin and the BMP pathways. Engagement of plasma membrane Wnt receptors LRP5 and LRP6 and the coreceptor FZD by Wnt leads to activation of DVL, which blocks a protein complex comprising axin, APC and GSK3. This allows the translocation of β-catenin to the nucleus, where it complexes with TCF/LEF1 and binds DNA. In addition, binding of BMP to its membrane receptor complex activates a signaling cascade involving heteromeric SMAD protein complexes, which translocate to the nucleus and bind DNA. Both of these transcriptional complexes then drive osteoblastogenic gene expression programs. DKK1 and sclerostin are endogenous proteins that inhibit osteoblast differentiation by binding to the LRP receptors and blocking Wnt docking. In addition, sclerostin binds BMPs and so can potentially block this pathway too.
APC, adenomatosis polyposis coli; BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; CK1, casein kinase 1; DKK1, Dickkopf1; DVL, human homolog of the Drosophila dishevelled gene; FZD, frizzled; GSK3, glycogen synthase kinase 3; LRP, LDL receptor-related protein; SMAD, TCF/LEF1, T cell factor/lymphoid enhancer binding factor 1.