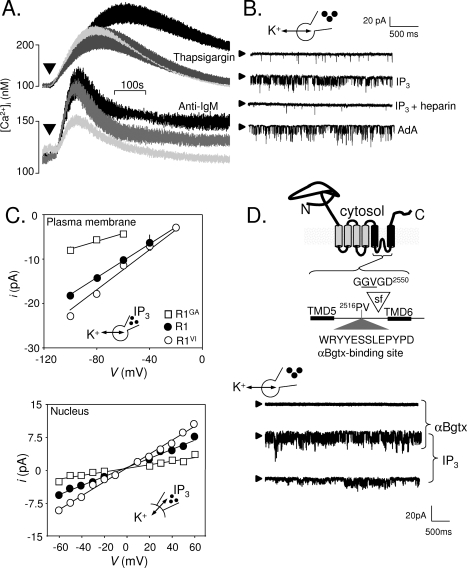
Figure 2.
Expression of IP3 receptors in the plasma membrane of DT40 cells. (A) Thapsigargin (top) stimulates Ca2+ release (palest line, in Ca2+-free medium) and Ca2+ entry (black line, in Ca2+-containing medium). The latter, SOCE, is entirely blocked by 300 nM GdCl3 (gray line). The bottom panel shows that activation of the BCR with anti-IgM stimulates both Ca2+ release and Ca2+ entry, but the latter is only partially inhibited by GdCl3. (B) Whole-cell patch-clamp recoding from DT40 cells with IP3, IP3 with heparin, or adenophostin A in the patch pipette. The holding potential was −100 mV; arrowheads denote the closed state. (C) Current−voltage (i−V) relationships for the IP3-stimulated currents recorded from the PM or nuclear envelope of DT40-KO cells stably transfected with wild-type IP3R1 (R1) or IP3R1 with mutants in the putative pore (G2547A, R1GA; V2548I, R1VI). The point mutations similarly affected γK of the IP3-activated currents in both settings. (D) The six TMDs of a single IP3R subunit are shown to highlight the putative selectivity filter (sf) and the engineered αBgtx-binding site. In whole-cell patch-clamp recordings from DT40 cells expressing IP3R1 with this αBgtx-binding site, intracellular IP3 stimulated channel openings, and both Po and γK were increased by extracellular αBgtx. Reproduced from ref (65) with permission. Copyright 2006. American Academy for the Advancement of Science.