Abstract
Background
Common FTO (fat mass and obesity associated) gene variants have recently been strongly associated with body mass index and obesity in several large studies. Here we set out to examine the association of the FTO variant rs9939609 with BMI in a 32 year follow up study of men born 1920-1924. Moreover, we analyzed the effect of physical activity on the different genotypes.
Methods
The FTO rs9936609 was genotyped using an Illumina golden gate assay. BMI was calculated using standard methods and body fat was estimated by measuring skinfold thickness using a Harpenden caliper. Physical activity was assessed using a four question medical questionnaire.
Results
FTO rs9939609 was genotyped in 1153 elderly Swedish men taking part of a population-based cohort study, the ULSAM cohort. The risk of obesity and differences in BMI according to genotype at the ages of 50, 60, 70, 77 and 82 were investigated. We found no increased risk of obesity and no association with BMI at any age with the FTO rs9939609 variant. We found however interaction between physical activity at the age of 50 years and genotype on BMI levels (p = 0.039) and there was a clear trend towards larger BMI differences between the TT and AA carriers as well as between AT and AA carriers in the less physically active subjects.
Conclusion
Here we found that the well established obesity risk allele for a common variant in FTO does not associate with increased BMI levels in a Swedish population of adult men which reached adulthood before the appearance of today's obesogenic enviroment. There is an interaction between physical activity and the effect of the FTO genotype on BMI levels suggesting that lack of physical activity is a requirement for an association of FTO gene variants to obesity.
Background
Today, about one in three adult can be classified as obese based on objectively measured weight and height. The increase started after the World War II, escalated in the seventies and the obesity rates have roughly tripled in the past 20 years. In Sweden one in hundred is considered morbidly obese today compared to one in thousand in the early 1970s [1-4].
This worldwide rapid increase in the prevalence of obesity is much due to environmental factors, such as a sedentary lifestyle and develops from an imbalance between energy ingested and expended [5]. It is however also widely accepted that obesity is under strong genetic control, explaining about 30% to 70% of the variation in BMI [6-8]. Recently, genome-wide association studies have led to rapid progress in our understanding of the genetic basis of various common diseases and a new candidate gene for obesity has been identified, the fat mass and obesity associated gene, FTO [9-14]. The first association between FTO and human obesity was found by Frayling et al. in early 2007 [10] in a genome wide association study of diabetes and has been positively replicated in additional studies involving several different ethnicities [15-21] including Swedish subjects [22,23]. These studies all show that subjects who are homozygous for the risk allele weigh about 3 kg more compared to those without the allele and individuals with the risk allele have about 1.5-fold increased risk of having obesity.
Most of the previous studies on the FTO gene variants are cross-sectional, leaving the longitudinal pattern of the associations between obesity and age-specific genetic effects poorly studied. Furthermore, the genetic effects on lifestyle factors and the effect in populations not affected by an obesogenic environment have not been thoroughly investigated. There have also been additional studies indicating that the genetic effects on BMI may be dependent on physical activity [24-26]. Four studies have so far analyzed if the effect of FTO on obesity in adults may be changed upon physical activity [23,27-29] with somewhat contrasting results. The aim of the present study was to address the issues of BMI and physical activity by assessing the genetic effects in a longitudinal cohort of adult men, the ULSAM cohort. The subjects were all born between 1920 and 1924, investigated at the age of 50 and reinvestigated at the ages of 60, 70, 77 and 82 years of age. The data available in Uppsala Longitudinal Study of Adult Men (ULSAM) allowed us to, compared to previous studies, investigate BMI differences at older ages and also highlight that the environment may play a fundamental role in the genetic contribution to increased BMI.
Methods
Subjects
In 1970, all men born between 1920 and 1924 and residing in Uppsala, Sweden, were invited to participate in a health survey, the Uppsala Longitudinal Study of Adult Men (ULSAM), described previously [30]. At baseline, at age 50 years, 2841 men were invited and 82% (n = 2322) accepted to participate. The subjects were then re-invited for examination at the ages of 60, 70, 77, and 82 years. At age 60, 2130 men were invited and 87% (1860) participated. At age 70, 1681 men were invited and 73% (1221) participated. The fourth examination was performed when the men were aged 77 years. At this time 748 of the 2322 participants at age 50 had died and another 176 men were ineligible for other reasons, leaving 1398 men possible subjects and 60% (839) attended the examination. At the last examination, at 82 years of age 971 men were invited and of the invited, 530 men (56%) participated in the examination. This study was approved by the Ethics committee of Uppsala University, Faculty of Medicine. All participants gave their written informed consent.
Anthropometry
The men were invited by a letter, which also explained the aim of the examination and all investigations were carried out under standardized conditions. Height was measured to the nearest whole centimeter and weight to the nearest whole kilogram. BMI was calculated as weight divided by height squared (kg/m2) and waist circumference in centimeter was measured midway between the lowest rib and the iliac crest in a supine position. The body fat was estimated by the measurement of skinfold thickness with a Harpenden caliper. The skinfold was measured to the nearest 0.2 mm on three different sides: on the back of the middle of the over-arm, just below the angle of the scapula and on the abdomen to the right of the umbilicus. All measurements were made with the subject in the sitting position.
Physical activity
DNA and information on self reported leisure time physical activity at age 50 years was available on 1860 men. This was estimated using four questions in a medical questionnaire. Based on these questions four different physical activity categories was constructed: sedentary, moderate, regular and athletic as described and validated previously [31-33].
Genotyping
Of the initial cohort of 2322 men, DNA was available from 1152 menobtained from examination at 70 and 77 years of age. The genotyping of FTO rs9939609 was carried out at the SNP technology platform at Uppsala University http://www.genotyping.SE/ using a Illumina Golden Gate Assay [34]. The SNP genotype call rate in the samples was 96.8%.
Statistical analysis
In order to test for deviation from Hardy-Weinberg equilibrium the Person's χ2 -test (1 d.f) was applied. Genotype and allele frequencies were calculated and logistic regression was used to calculate odds ratio (OR) with a 95% confidence interval (CI) assuming an additive model. Association with overweight and obesity was determined comparing subjects with normal weight (BMI < 25 kg/m2 ) and overweight (BMI ≥ 25 kg/m2 ) and subjects with normal weight (BMI < 25 kg/m2 ) and obesity (BMI ≥ 30 kg/m2 ), respectively. Quantitative skewed variables were log-transformed before analysis. Associations between genotypes and phenotypes were analyzed with linear regression, assuming an additive and dominant model. The effect of physical activity on the impact of the FTO rs9939609 on BMI levels was analyzed with an ANOVA test, assuming an additive model and an interaction parameter was included to test for interaction effects. Association between FTO rs9939609 and BMI across different age groups was analyzed with linear models and only subjects with available data at age 82 were included in the analysis. P-values < 0.05 were considered significant. All the analysis was performed using PLINK http://pngu.mgh.harvard.edu/purcell/plink/[35] and Graphpad Prism version 4.03 (PraphPad Software, San Diego, USA).
Results
The obesity associated FTO variant rs9939609 was genotyped in the ULSAM cohort, a longitudinal cohort of adult men. Descriptive characteristics at baseline of the cohort are presented in Table 1. Of the total number of subjects 631 (55%) were considered normal weight, 521 (41%) had a BMI over 25 kg/m2 and 55 (4.8%) had a BMI over 30 kg/m2 and were considered overweight and obese, respectively. The mean BMI among all subjects was 24.8 ± 2.9 kg/m2 and among the normal weight 22.8 ± 1.5 kg/m2 . Among the overweight and obese the mean BMI was 27.3 ± 2.1 kg/m2 and 32.1 ± 1.9 kg/m2 , respectively.
Table 1.
Descriptive characteristics of all subjects included in the analysis at 50 years.
| Characteristics | All | Normal weight | Overweight | Obese |
|---|---|---|---|---|
| N | 1152 | 631 | 521 | 55 |
| Weight (kg) | 77.4 ± 10.0 | 71.4 ± 6.8 | 84.7 ± 8.2 | 100.1 ± 10.3 |
| Length (m) | 176.5 ± 5 8 | 176.9 ± 5.8 | 176.0 ± 5.7 | 175.6 ± 6.4 |
| BMI (kg/m2 ) | 24.8 ± 2.9 | 22.8 ± 1.5 | 27.3 ± 2.1 | 32.1 ± 1.9 |
Values are geometric means ± SD.
The analysis on FTO rs9939609 showed an allele frequency similar as reported by Frayling et al [9] with a minor allele frequency of 37% among the normal-weight subjects, 40% and 37% among overweight and obese subjects, respectively. The allelic odds ratio for rs9939609 on the risk of being overweight and obese compared to being of normal weight was estimated comparing subjects with normal weight (BMI < 25 kg/m2 ) and overweight (BMI ≥ 25 kg/m2 ) and subjects with normal weight (BMI < 25 kg/m2 ) and obesity (BMI ≥ 30 kg/m2 ), assuming an additive model but no association was found in either groups (Table 2).
Table 2.
Association study of FTO rs9939609 variant with overweight and obesity
| Group | n | Genotype, n (%) | MAF, % | OR (95% CI) | P | HWE | ||
|---|---|---|---|---|---|---|---|---|
| TT | TA | AA | ||||||
| Normal weight | 607 | 242 (40) | 277 (46) | 88 (14) | 37 | 0.544 | ||
| Overweight | 454 | 169 (37) | 206 (45) | 79 (17) | 40 | 1.124 (0.942-1.341) | 0.194 | 0.242 |
| Obese | 54 | 16 (30) | 29 (54) | 9 (17) | 44 | 1.294 (0.870-1.927) | 0.203 | 0.588 |
Data are number of subjects in each group, at age 50 years, and number of subjects for each genotype (% in each group). Minor allele frequency (MAF) for each group is given in percentage. Odds ratio (OR) with a 95% confidence interval (CI) was calculated assuming an additive model. Association with overweight and obesity was determined comparing subjects with normal weight (BMI < 25 kg/m2 ) and overweight (BMI = 25 kg/m2 ) and subjects with normal weight (BMI < 25 kg/m2 ) and obesity (BMI ≥ 30 kg/m2 ), respectively. HWE indicate p-values for deviation from Hardy-Weinberg Equilibrium.
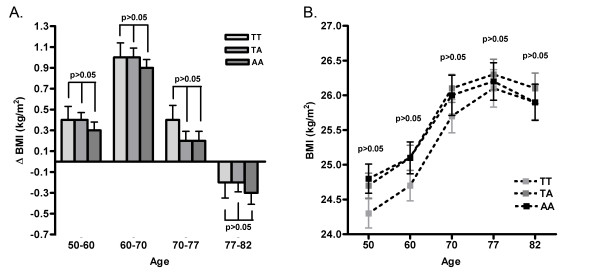
We further investigated the association between rs9939609 and BMI as well as other measurements of obesity such as waist circumference and skinfold thickness at baseline for all subjects as well as BMI and waist circumference at 60, 70, 77 and 82 years of age. No association at any age was found between the minor A-allele and BMI, or any of the other obesity measurements (Table 3, Figure 1).
Table 3.
Anthropometric characteristics stratified according to FTO rs9939609genotypes at different ages.
| TT | TA | AA | PAdd | PDom | |
|---|---|---|---|---|---|
| Age 50 | |||||
| N = 1115 | 426 | 512 | 177 | ||
| Weight (kg) | 76.8 ± 9.4 | 77.8 ± 10.5 | 78.0 ± 10.7 | 0.309 | 0.192 |
| Length (m) | 176.1 ± 5.9 | 176.8 ± 5.5 | 176.5 ± 5.8 | 0.235 | 0.072 |
| BMI (kg/m2 ) | 24.3 ± 2.8 | 24.7 ± 2.9 | 24.8 ± 3.2 | 0.130 | 0.159 |
| Waist circumference (cm) | 86.0 ± 8.3 | 87.3 ± 8.6 | 88.6 ± 8.7 | 0.189 | 0.323 |
| Skinfold abdominal (mm) | 19.2 ± 9.2 | 19.5 ± 9.2 | 19.4 ± 9.5 | 0.538 | 0.949 |
| Skinfold subscapular (mm) | 16.2 ± 5.7 | 15.8 ± 5.6 | 17.0 ± 6.9 | 0.857 | 0.246 |
| Skinfold triceps (mm) | 10.2 ± 3.9 | 10.3 ± 3.8 | 10.2 ± 3.9 | 0.282 | 0.243 |
| Age 60 | |||||
| N = 1051 | 404 | 479 | 168 | ||
| Weight (kg) | 77.4 ± 10.2 | 79 0 ± 11.0 | 78.9 ± 11.8 | 0.169 | 0.171 |
| Length (m) | 175.7 ± 5.9 | 176.4 ± 5.5 | 176.0 ± 5.9 | 0.303 | 0.121 |
| BMI (kg/m2 ) | 24.7 ± 2.9 | 25.1 ± 3.2 | 25.1 ± 3.4 | 0.147 | 0.111 |
| Waist circumference (cm) | 89.5 ± 9.5 | 90.3 ± 9.8 | 89.9 ± 10.2 | 0.383 | 0.159 |
| Skinfold abdominal (mm) | 26.7 ± 11.2 | 26.8 ± 10.5 | 26.7 ± 11.2 | 0.624 | 0.697 |
| Skinfold subscapular (mm) | 18.5 ± 7.1 | 17.7 ± 7.2 | 18.7 ± 7.1 | 0.739 | 0.586 |
| Skinfold triceps (mm) | 10.1 ± 5.2 | 10.4 ± 6.4 | 9.9 ± 5.7 | 0.879 | 0.978 |
| Age 70 | |||||
| N = 1056 | 401 | 490 | 165 | ||
| Weight (kg) | 78.8 ± 10.2 | 81.1 ± 11.9 | 80.2 ± 12.7 | 0.842 | 0.814 |
| Length (m) | 174.6 ± 5.9 | 175.2 ± 5.6 | 174.9 ± 6.1 | 0.259 | 0.116 |
| BMI (kg/m2 ) | 25.7 ± 3.1 | 26.1 ± 3.5 | 26.0 ± 3.6 | 0.426 | 0.787 |
| Waist circumference (cm) | 94.0 ± 8.9 | 95.3 ± 9.8 | 94.7 ± 10.2 | 0.266 | 0.203 |
| Age 77 | |||||
| N = 749 | 287 | 342 | 120 | ||
| Weight (kg) | 77.8 ± 11.2 | 79.8 ± 11.5 | 79.6 ± 11.9 | 0.813 | 0.804 |
| Length (m) | 173.5 ± 5.7 | 173.8 ± 5.6 | 173.5 ± 6.0 | 0.849 | 0.623 |
| BMI (kg/m2 ) | 26.1 ± 3.5 | 26.3 ± 3.5 | 26.2 ± 3.6 | 0.146 | 0.085 |
| Waist circumference (cm) | 94.9 ± 9.7 | 95.9 ± .9.9 | 96.1 ± 10.7 | 0.366 | 0.341 |
| Age 82 | |||||
| N = 493 | 193 | 230 | 70 | ||
| Weight (kg) | 77.4 ± 10.8 | 78.2 ± 11.3 | 76.0 ± 11.7 | 0.548 | 0.963 |
| Length (m) | 173.3 ± 5.6 | 173.0 ± 5.6 | 171.9 ± 5.1 | 0.102 | 0.295 |
| BMI (kg/m2 ) | 25.9 ± 3.5 | 26.1 ± 3.3 | 25.9 ± 3.7 | 0.259 | 0.225 |
| Waist circumference (cm) | 95.8 ± .9.7 | 95.6 ± 9.3 | 95.7 ± 11.2 | 0.902 | 0.851 |
Values are geometric means ± SD. Skewed variables were log-transformed and analyzed with linear regression, assuming an additive and dominant model.
Figure 1.
A: Associations between FTO rs9939609 and BMI across different ages; 50, 60, 70, 77 and 82 years. B: Changes in BMI levels between different ages (50-60, 60-70, 70-77 and 77-82 years) and according to FTO rs9939609 genotype.
The longitudinal pattern of the associations between obesity and age-specific genetic effects was analyzed in 32-years follow up. Although not significant, we observed a trend toward decreasing differences in BMI according to genotype at older ages, as was also observed by [36](Figure 1A). We also investigated the longitudinal changes in BMI from the age of 50 years to 82 years (Figure 1B). We observed an increase in BMI levels from 50-77 years, with the highest increase between 60-70 years, and a decrease in BMI from 77-82. However, neither the A or T allele at any age were associated with a higher increase or decrease in BMI which means that the variant does not seem to affect the changes in BMI levels at any stage in older life.
From a medical questionnaire we had access to self-reported leisure time physical activity at age 50 years which divided the subjects into four categories. 15% of all subjects were considered sedentary, 36% had moderate physical activity, 44% had regular physical activity and 5% were considered athletic, and as expected subjects that were considered athletic had lower BMI levels compared to the less active subjects (p < 0.01). The subjects, based on the physical activity, were further stratified according to the FTO rs9939609 genotype (Figure 2A). Physical activity influence the effect of FTO rs9939609 on BMI (Pinteraction = 0.039) across the four different categories. The BMI per FTO risk allele was higher in sedentary active (0.33 per risk allele, SD 0.39) and moderate active individuals (0.13 per risk allele, SD 0.21) compared with the BMI among individuals with regular activity (-0.25 per risk allele, SD 0.18) and athletic individuals (-0.29 per risk allele, SD 0.47). The difference in BMI per allele in each category was however not significant different (P > 0.05 for all categories) (Figure 2B).
Figure 2.
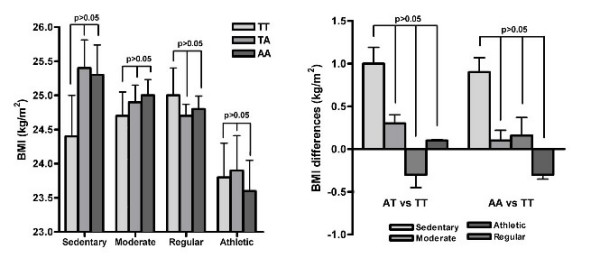
Effect of physical activity on the impact of the FTO rs9939609 variant on BMI levels at age 50 years. Subjects were divided into four different categories based on self-reported leisure time physical activity: sedentary, moderate, regular and athletic. A: BMI levels for each category stratified according to FTO rs9939609 genotype, data are means and ± SEM B: Differences in BMI levels for the different categories between heterozygotes and homozygotes T-allele carriers and between heterozygotes and homozygotes A-allele carriers, respectively.
Discussion
There has been a considerable increase in obesity from 6% in 1963 to 11% in 1993 in Sweden [2,37]. This increase has continued and there has been a twofold increase during the last two decades [3,4,38]. The men included in the ULSAM cohort were all examined 1970-74 at the age of 50 years, when the prevalence of moderate obesity were very low, 0.8% compared to 3.8% in 2000-2005 [4]. Moreover, these men have lower BMI levels compared to the cohorts previously used to study the FTO gene. The mean BMI was 24.5 in the ULSAM cohort compared to the mean BMI of 26.5 in the cohorts of older adult reported by Frayling et al. [9]. The ULSAM cohort provides thus an important opportunity to study genetic components in a lean population that lived in a less obesogenic environment compared with today. Our results indicate that the impact of FTO seems less prominent in this lean population since there was no significant difference in BMI for the carriers of the risk allele, not at baseline or at any of the other ages that we studied. Neither did we find any association for other measurements of obesity such as waist circumference and skinfold thickness at any age. It is however important that these findings are replicated in several lean populations in order to be able to draw any conclusions.
We further analyzed the longitudinal pattern of the associations between BMI and FTO variants. So far, Frayling et al. [9] and Qi et al. [36] have observed a trend toward decreasing associations between FTO variants and BMI at older age. Qi et al. further showed that this was found in men, whereas the associations were constant across different age groups in women [36]. Although we did not reach significance, our data support these findings. The small effect of rs9939609 on BMI in our cohort at baseline was not seen at all at older ages. Thus, it appears that the association between the SNP and obesity risk declined with older age especially in men.
Studies on BMI changes in relation to physical activity and genetic factors have shown that both play a significant independent role in weight changes. However, there has been additional evidence of a gene-environment interaction in men, indicating that genetic effects on weight gain may be more highly dependent on physical activity level [24-26]. Four studies have so far analyzed if the effect of FTO on obesity in adults may be changed upon physical activity [27,23,29] with somewhat contrasting results. Andreasen et al. [27] demonstrated that the impact of the FTO rs9939609 genotype was influenced by the habitual level of physical activity in a population based study on Danish subjects with mean BMI of 26 kg/m2 . These subjects were divided according to self-reported physically activity into passive, light or medium physically active and hard or very hard physically active, and stratified according to FTO genotype. The study showed that physical inactive homozygous FTO rs9939609 A-allele carriers had around 2 kg/m2 higher BMI compared to inactive homozygous T-allele carriers [27]. These findings were supported by Cauchi et al [29] and Rampersaud et al. [28] for other FTO variants. Both showed that the differences in BMI were larger in physical inactive subjects while no difference was found at all in physical active subjects. However, a large study by Jonsson et al. [23] on Swedish adults did not find any interaction between rs9939609 and physical activity on BMI. The biggest differences between these studies is that the Swedish subjects had a mean BMI of 24.3 kg/m2 and were leaner compared to the other cohorts and physical activity was only based on two categories, active or not active. Our results showed the presence of an interaction and support the suggestion that the increased levels of BMI related to FTO variants may be less prominent if the persons are physical active. Our interaction is however weaker compared to the other studies and the effect size is smaller. The lower effect size in our study compared to previous studies may be due to a leaner cohort. We have furthermore older subjects compared to other studies which may affect the interaction and weaken the effect of physical activity. Furthermore, since the categories of physical activity in our study have been assessed by questionnaire under- or over-reporting may occur which may also influence the result. Our finding is however consistent with previous studies and suggests that inherited factors significantly influence body weight in sedentary subjects and that in physically more active individuals the effect of the FTO gene seem to diminished, further suggesting that high physical activity would be particularly beneficial in those genetically predisposed to obesity due to the FTO gene. As the gene expression might vary due to differences in the environment it is interesting to study the effect of FTO in a population with lower mean BMI than has previously been studied. Furthermore, several of the susceptibility genes to common diseases do not have a primary causal role in the predisposition to disease without considering environmental factors. Rather, susceptibility genes may act as responders, or modifiers, to factors such as stress, environment, as well as physical inactivity. Fisher et al. [39] has also demonstrated that loss of the mouse Fto gene product reduces adiposity, and that even moderate reduction in Fto expression protects from diet-induced obesity which makes it tempting to speculate that physical activity may reduce the FTO gene expression and influence the interacting genes that in turn modify energy homeostasis.
Conclusion
Here we set out to examine the association of the FTO variant rs9939609 with BMI in a longitudinal cohort of adult men. We could not find any association between FTO variant and obesity or obesity measurements at any of the ages studied. We further analyzed the effect of physical activity on BMI levels and found an interaction between rs9939609 and level of activity and saw that inactive people carriers of the A-allele had higher BMI-values than non-carriers. This, together with our lack of association with BMI in our lean population, indicate that FTO seem to have a greater effect on those that are less physical active and with higher BMI. Thus, these results are an example of environmental influences and that inactivity is a potential vital environmental trigger for disease susceptibility dependent on the FTO genotype.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JJ carried out the study, analyzed the data, participated in the design, and drafted the manuscript. TA performed the genotyping, participated in the genetic analysis and in the writing of the manuscript. UR and LL participated in analysis of the phenotypes and in writing of the manuscript. RF and HS conceived the study, participated in the design and writing of the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Josefin A Jacobsson, Email: Josefin.Jacobsson@neuro.uu.se.
Ulf Risérus, Email: Ulf.Riserus@pubcare.uu.se.
Tomas Axelsson, Email: Tomas.Axelsson@medsci.uu.se.
Lars Lannfelt, Email: Lars.Lannfelt@pubcare.uu.se.
Helgi B Schiöth, Email: helgi.schioth@neuro.uu.se.
Robert Fredriksson, Email: robert.fredriksson@neuro.uu.se.
Acknowledgements
The study was supported by the Swedish Research Council (VR), AFA insurance, Svenska Läkarsällskapet, Åhlens Foundation, The Novo Nordisk Foundation, Swedish Royal Academy of Sciences, Borgströms Stiftelse, The Göran Gustafsson Foundation and Magnus Bergvall Foundation. The SNP genotyping was performed by the SNP Technology Platform, Uppsala, Sweden http://www.genotyping.se with support from Uppsala University and the Knut and Alice Wallenberg foundation.
References
- Sturm R. Increases in morbid obesity in the USA: 2000-2005. Public Health. 2007;121:492–496. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren A, Eriksson H, Larsson B, Svardsudd K, Tibblin G, Welin L, Wilhelmsen L. Secular changes in cardiovascular risk factors over 30 years in Swedish men aged 50: the study of men born in 1913, 1923, 1933 and 1943. J Intern Med. 2000;247:111–118. doi: 10.1046/j.1365-2796.2000.00589.x. [DOI] [PubMed] [Google Scholar]
- Neovius M, Janson A, Rossner S. Prevalence of obesity in Sweden. Obes Rev. 2006;7:1–3. doi: 10.1111/j.1467-789x.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- Neovius M, Teixeira-Pinto A, Rasmussen F. Shift in the composition of obesity in young adult men in Sweden over a third of a century. Int J Obes (Lond) 2008;32:832–836. doi: 10.1038/sj.ijo.0803784. [DOI] [PubMed] [Google Scholar]
- Eckel RH. Obesity and heart disease: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1997;96:3248–3250. doi: 10.1161/01.cir.96.9.3248. [DOI] [PubMed] [Google Scholar]
- Loos RJ, Bouchard C. Obesity--is it a genetic disorder? J Intern Med. 2003;254:401–425. doi: 10.1046/j.1365-2796.2003.01242.x. [DOI] [PubMed] [Google Scholar]
- Bellisari A. Evolutionary origins of obesity. Obes Rev. 2008;9:165–180. doi: 10.1111/j.1467-789X.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- Marti A, Moreno-Aliaga MJ, Hebebrand J, Martinez JA. Genes, lifestyles and obesity. Int J Obes Relat Metab Disord. 2004;28(Suppl 3):S29–36. doi: 10.1038/sj.ijo.0802808. [DOI] [PubMed] [Google Scholar]
- Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina C, Meyre D, Gallina S, Durand E, Korner A, Jacobson P, Carlsson LM, Kiess W, Vatin V, Lecoeur C, Delplanque J, Vaillant E, Pattou F, Ruiz J, Weill J, Levy-Marchal C, Horber F, Potoczna N, Hercberg S, Le Stunff C, Bougneres P, Kovacs P, Marre M, Balkau B, Cauchi S, Chevre JC, Froguel P. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orru M, Usala G, Dei M, Lai S, Maschio A, Busonero F, Mulas A, Ehret GB, Fink AA, Weder AB, Cooper RS, Galan P, Chakravarti A, Schlessinger D, Cao A, Lakatta E, Abecasis GR. Genome-Wide Association Scan Shows Genetic Variants in the FTO Gene Are Associated with Obesity-Related Traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyre D, Delplanque J, Chevre JC, Lecoeur C, Lobbens S, Gallina S, Durand E, Vatin V, Degraeve F, Proenca C, Gaget S, Korner A, Kovacs P, Kiess W, Tichet J, Marre M, Hartikainen AL, Horber F, Potoczna N, Hercberg S, Levy-Marchal C, Pattou F, Heude B, Tauber M, McCarthy MI, Blakemore AI, Montpetit A, Polychronakos C, Weill J, Coin LJ. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet. 2009;41:157–159. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I, Soranzo N, Tanaka T, Timpson NJ, Almgren P, Bennett A. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, Styrkarsdottir U, Gretarsdottir S, Thorlacius S, Jonsdottir I, Jonsdottir T, Olafsdottir EJ, Olafsdottir GH, Jonsson T, Jonsson F, Borch-Johnsen K, Hansen T, Andersen G, Jorgensen T, Lauritzen T, Aben KK, Verbeek AL, Roeleveld N, Kampman E, Yanek LR, Becker LC, Tryggvadottir L, Rafnar T, Becker DM, Gulcher J. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- Peters T, Ausmeier K, Ruther U. Cloning of Fatso (Fto), a novel gene deleted by the Fused toes (Ft) mouse mutation. Mamm Genome. 1999;10:983–986. doi: 10.1007/s003359901121. [DOI] [PubMed] [Google Scholar]
- Hunt SC, Stone S, Xin Y, Scherer CA, Magness CL, Iadonato SP, Hopkins PN, Adams TD. Association of the FTO gene with BMI. Obesity (Silver Spring) 2008;16:902–904. doi: 10.1038/oby.2007.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta K, Nakata Y, Matsuo T, Kamohara S, Kotani K, Komatsu R, Itoh N, Mineo I, Wada J, Masuzaki H, Yoneda M, Nakajima A, Miyazaki S, Tokunaga K, Kawamoto M, Funahashi T, Hamaguchi K, Yamada K, Hanafusa T, Oikawa S, Yoshimatsu H, Nakao K, Sakata T, Matsuzawa Y, Tanaka K, Kamatani N, Nakamura Y. Variations in the FTO gene are associated with severe obesity in the Japanese. J Hum Genet. 2008;53:546–553. doi: 10.1007/s10038-008-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Liu PH, Lee WJ, Chang TJ, Jiang YD, Li HY, Kuo SS, Lee KC, Chuang LM. Common variation in the fat mass and obesity-associated (FTO) gene confers risk of obesity and modulates BMI in the Chinese population. Diabetes. 2008;57:2245–2252. doi: 10.2337/db08-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha SW, Choi SM, Kim KS, Park BL, Kim JR, Kim JY, Shin HD. Replication of genetic effects of FTO polymorphisms on BMI in a Korean population. Obesity (Silver Spring) 2008;16:2187–2189. doi: 10.1038/oby.2008.314. [DOI] [PubMed] [Google Scholar]
- Villalobos-Comparan M, Teresa Flores-Dorantes M, Teresa Villarreal-Molina M, Rodriguez-Cruz M, Garcia-Ulloa AC, Robles L, Huertas-Vazquez A, Saucedo-Villarreal N, Lopez-Alarcon M, Sanchez-Munoz F, Dominguez-Lopez A, Gutierrez-Aguilar R, Menjivar M, Coral-Vazquez R, Hernandez-Stengele G, Vital-Reyes VS, Acuna-Alonzo V, Romero-Hidalgo S, Ruiz-Gomez DG, Riano-Barros D, Herrera MF, Gomez-Perez FJ, Froguel P, Garcia-Garcia E, Teresa Tusie-Luna M, Aguilar-Salinas CA, Canizales-Quinteros S. The FTO gene is associated with adulthood obesity in the Mexican population. Obesity (Silver Spring) 2008;16:2296–2301. doi: 10.1038/oby.2008.367. [DOI] [PubMed] [Google Scholar]
- Cornes BK, Lind PA, Medland SE, Montgomery GW, Nyholt DR, Martin NG. Replication of the association of common rs9939609 variant of FTO with increased BMI in an Australian adult twin population but no evidence for gene by environment (G × E) interaction. Int J Obes (Lond) 2009;33:75–79. doi: 10.1038/ijo.2008.223. [DOI] [PubMed] [Google Scholar]
- Jacobsson JA, Danielsson P, Svensson V, Klovins J, Gyllensten U, Marcus C, Schioth HB, Fredriksson R. Major gender difference in association of FTO gene variant among severely obese children with obesity and obesity related phenotypes. Biochem Biophys Res Commun. 2008;368:476–482. doi: 10.1016/j.bbrc.2008.01.087. [DOI] [PubMed] [Google Scholar]
- Jonsson A, Renstrom F, Lyssenko V, Brito EC, Isomaa B, Berglund G, Nilsson PM, Groop L, Franks PW. Assessing the effect of interaction between an FTO variant (rs9939609) and physical activity on obesity in 15,925 Swedish and 2,511 Finnish adults. Diabetologia. 2009. [DOI] [PubMed]
- Heitmann BL, Kaprio J, Harris JR, Rissanen A, Korkeila M, Koskenvuo M. Are genetic determinants of weight gain modified by leisure-time physical activity? A prospective study of Finnish twins. Am J Clin Nutr. 1997;66:672–678. doi: 10.1093/ajcn/66.3.672. [DOI] [PubMed] [Google Scholar]
- Mustelin L, Silventoinen K, Pietilainen K, Rissanen A, Kaprio J. Physical activity reduces the influence of genetic effects on BMI and waist circumference: a study in young adult twins. Int J Obes (Lond) 2009;33:29–36. doi: 10.1038/ijo.2008.258. [DOI] [PubMed] [Google Scholar]
- Karnehed N, Tynelius P, Heitmann BL, Rasmussen F. Physical activity, diet and gene-environment interactions in relation to body mass index and waist circumference: the Swedish young male twins study. Public Health Nutr. 2006;9:851–858. doi: 10.1017/PHN2005926. [DOI] [PubMed] [Google Scholar]
- Andreasen CH, Stender-Petersen KL, Mogensen MS, Torekov SS, Wegner L, Andersen G, Nielsen AL, Albrechtsen A, Borch-Johnsen K, Rasmussen SS, Clausen JO, Sandbaek A, Lauritzen T, Hansen L, Jorgensen T, Pedersen O, Hansen T. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57:95–101. doi: 10.2337/db07-0910. [DOI] [PubMed] [Google Scholar]
- Rampersaud E, Mitchell BD, Pollin TI, Fu M, Shen H, O'Connell JR, Ducharme JL, Hines S, Sack P, Naglieri R, Shuldiner AR, Snitker S. Physical activity and the association of common FTO gene variants with body mass index and obesity. Arch Intern Med. 2008;168:1791–1797. doi: 10.1001/archinte.168.16.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauchi S, Stutzmann F, Cavalcanti-Proenca C, Durand E, Pouta A, Hartikainen AL, Marre M, Vol S, Tammelin T, Laitinen J, Gonzalez-Izquierdo A, Blakemore AI, Elliott P, Meyre D, Balkau B, Jarvelin MR, Froguel P. Combined effects of MC4R and FTO common genetic variants on obesity in European general populations. J Mol Med. 2009;87:537–546. doi: 10.1007/s00109-009-0451-6. [DOI] [PubMed] [Google Scholar]
- Hedstrand H. A study of middle-aged men with particular reference to risk factors for cardiovascular disease. Ups J Med Sci Suppl. 1975;19:1–61. [PubMed] [Google Scholar]
- Byberg L, Zethelius B, McKeigue PM, Lithell HO. Changes in physical activity are associated with changes in metabolic cardiovascular risk factors. Diabetologia. 2001;44:2134–2139. doi: 10.1007/s001250100022. [DOI] [PubMed] [Google Scholar]
- Michaelsson K, Olofsson H, Jensevik K, Larsson S, Mallmin H, Berglund L, Vessby B, Melhus H. Leisure physical activity and the risk of fracture in men. PLoS Med. 2007;4:e199. doi: 10.1371/journal.pmed.0040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman A, Byberg L, Reneland R, Lithell HO. Muscle morphology, self-reported physical activity and insulin resistance syndrome. Acta Physiol Scand. 2002;175:325–332. doi: 10.1046/j.1365-201X.2002.01000.x. [DOI] [PubMed] [Google Scholar]
- Fan JB, Oliphant A, Shen R, Kermani BG, Garcia F, Gunderson KL, Hansen M, Steemers F, Butler SL, Deloukas P, Galver L, Hunt S, McBride C, Bibikova M, Rubano T, Chen J, Wickham E, Doucet D, Chang W, Campbell D, Zhang B, Kruglyak S, Bentley D, Haas J, Rigault P, Zhou L, Stuelpnagel J, Chee MS. Highly parallel SNP genotyping. Cold Spring Harb Symp Quant Biol. 2003;68:69–78. doi: 10.1101/sqb.2003.68.69. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Kang K, Zhang C, van Dam RM, Kraft P, Hunter D, Lee CH, Hu FB. Fat mass-and obesity-associated (FTO) gene variant is associated with obesity: longitudinal analyses in two cohort studies and functional test. Diabetes. 2008;57:3145–3151. doi: 10.2337/db08-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissner L, Johansson SE, Qvist J, Rossner S, Wolk A. Social mapping of the obesity epidemic in Sweden. Int J Obes Relat Metab Disord. 2000;24:801–805. doi: 10.1038/sj.ijo.0801237. [DOI] [PubMed] [Google Scholar]
- Sundquist K, Qvist J, Johansson SE, Sundquist J. Increasing trends of obesity in Sweden between 1996/97 and 2000/01. Int J Obes Relat Metab Disord. 2004;28:254–261. doi: 10.1038/sj.ijo.0802553. [DOI] [PubMed] [Google Scholar]
- Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Bruning JC, Ruther U. Inactivation of the Fto gene protects from obesity. Nature. 2009;458:894–898. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]