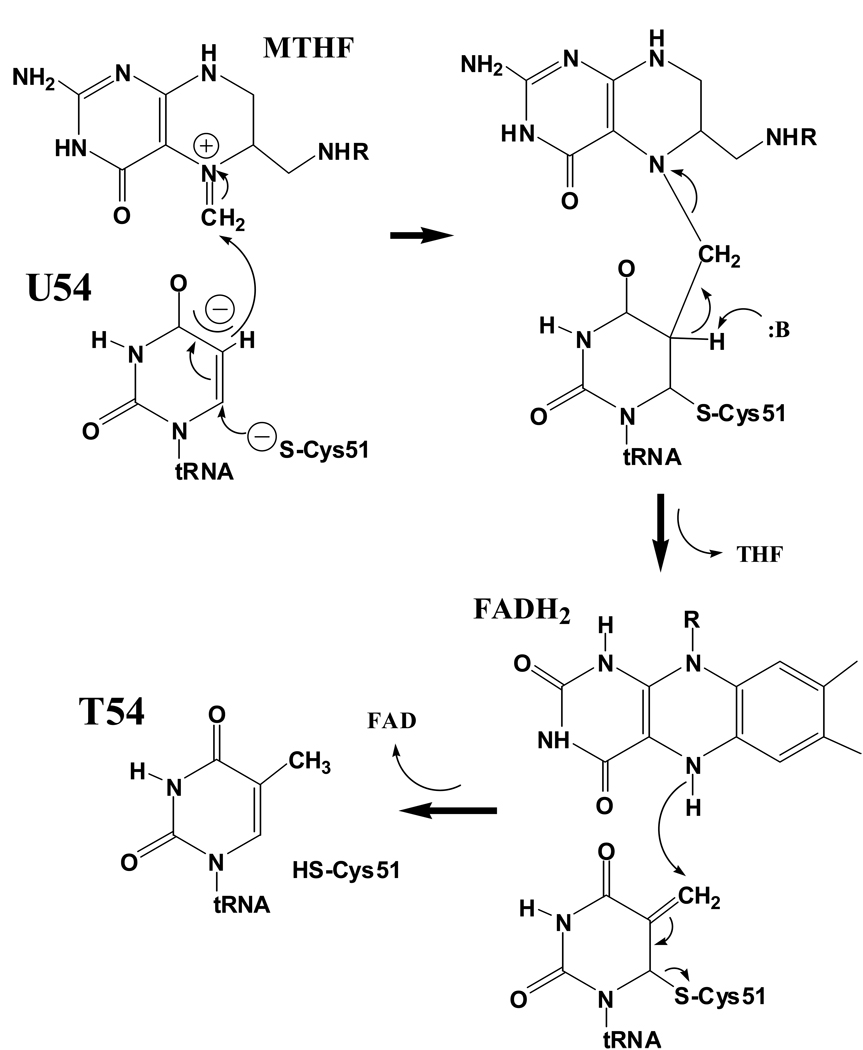
Figure 2.
Proposed mechanism for T. thermophilus TrmFO-catalyzed formation of ribothymidine at position 54 of tRNA. Nucleophilic attack on C6 generates in turn a nucleophilic C5 position that attacks the methylene group of 5,10-methylenetetrahydrofolate (MTHF). The covalent intermediate (upper right) is resolved by base abstraction of a proton at C5, analogous to the TrmA mechanism depicted in Figure 1A, but producing a methylene group at C5. The methylene is reduced to a methyl group by a redox chain involving both FAD and NADP+ cofactors.