Figure 1.
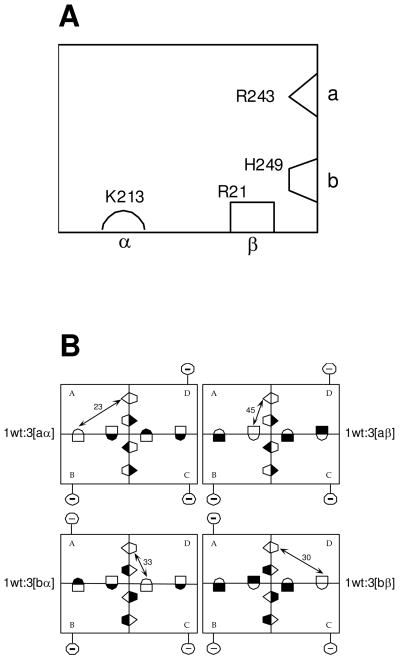
(A) Schematic representation of a single subunit of E. coli PFK. The two complementary halves of each active site are labeled ‘a’ and ‘b’ respectively, while the two complementary halves of each allosteric site are labeled ‘α’ and ‘β’ respectively. Residues are depicted in each half-site which, when modified to glutamate (or alanine in the case of R21), greatly diminish either Fru-6-P or PEP binding to the entire active site or allosteric site, respectively. (B) A schematic for the four hybrid tetramers that isolate each of the four unique heterotropic interactions contained in wild-type EcPFK. Allosteric sites are depicted as lying along the horizontal dimer-dimer interface, while the Fru-6-P portion of the active site lies along the vertical dimer-dimer interface. Each hybrid is obtained from the 1:3 combination of a wildtype parent and one of 4 modified parent proteins, the latter designated aα, aβ, bα, bβ respectively depending on the location of the mutations, as described in A, and denoted with a filled half-binding site symbol. Reference to X-ray structures allows the unambiguous determination of the relationship between the single unmodified substrate site and single unmodified allosteric site that remain in each 1:3 hybrid. Purification of 1:3 hybrids is facilitated by the changes introduced in 2 charged residues located on the surface of the modified parent proteins, depicted in the figure by the circled negative sign connected to each modified subunit. Details can be obtained from reference (4) from which these figures were taken.