Abstract
During mating in many butterfly species, males transfer spermatophores that contain anti-aphrodisiacs to females that repel conspecific males. For example, males of the large cabbage white, Pieris brassicae (Lepidoptera: Pieridae), transfer the anti-aphrodisiac, benzyl cyanide (BC) to females. Accessory reproductive gland (ARG) secretion of a mated female P. brassicae that is deposited with an egg clutch contains traces of BC, inducing Brussels sprouts plants (Brassica oleracea var. gemmifera) to arrest certain Trichogramma egg parasitoids. Here, we assessed whether deposition of one egg at a time by the closely related small cabbage white, Pieris rapae, induced B. oleracea var. gemmifera to arrest Trichogramma wasps, and whether this plant synomone is triggered by substances originating from male P. rapae seminal fluid. We showed that plants induced by singly laid eggs of P. rapae arrest T. brassicae wasps three days after butterfly egg deposition. Elicitor activity was present in ARG secretion of mated female butterflies, whereas the secretion of virgin females was inactive. Pieris rapae used a mixture of methyl salicylate (MeSA) and indole as an anti-aphrodisiac. We detected traces of both anti-aphrodisiacal compounds in the ARG secretion of mated female P. rapae, whereas indole was lacking in the secretion of virgin female P. rapae. When applied onto the leaf, indole induced changes in the foliar chemistry that arrested T. brassicae wasps. This study shows that compounds of male seminal fluid incur possible fitness costs for Pieris butterflies by indirectly promoting egg parasitoid attack.
Keywords: Induced indirect plant defense, Egg deposition, Pieris rapae, Trichogramma, Brassica, Indole, Methyl salicylate, Elicitor
Introduction
In arthropods, males are known to transfer materials during mating, typically in the form of protein-rich spermatophores (Gwynne 2008). Many butterfly males transfer an ejaculate to a female’s bursa copulatrix that consists of sperm, nutrients, hormones, and anti-aphrodisiacs, (pheromone compounds that repel subsequent males) (Boggs and Gilbert 1979; Boggs 1990). By being unattractive to other males, mated females labeled with an anti-aphrodisiac are relieved of harassment during oviposition. However, utilization of anti-aphrodisiacs may incur fitness costs to both females and males when natural enemies exploit them. Parasitic wasps, including egg parasitoids, often exploit the sexual communication system of their hosts during foraging (reviewed by Vinson 1984; Stowe et al. 1995; Powell 1999; Fatouros et al. 2008b).
Recently, we showed that Trichogramma egg parasitoids utilize the anti-aphrodisiac of one of their hosts, the gregarious large cabbage white butterfly, Pieris brassicae L. (Fatouros et al. 2005b; Huigens et al. 2009). Male P. brassicae induce female refractoriness for mating by transferring the anti-aphrodisiac, benzyl cyanide (BC), within the ejaculate (Andersson et al. 2003). Benzyl cyanide acts as a kairomone for Trichogramma brassicae Bezdenko and T. evanescens Westwood by attracting the wasps to mated P. brassicae females, thus facilitating phoretic transport to host oviposition sites (Fatouros et al. 2005b; Huigens et al. 2009). In addition, BC incurs another indirect cost on P. brassicae by triggering the release of plant compounds that arrest T. brassicae wasps, egg parasitoids of the butterfly (Fatouros et al. 2005a, 2008a).
Plants fed or oviposited upon by herbivores often release chemical cues (i.e., plant synomones) that attract predators and/or parasitoids, enemies of the herbivorous attackers (Hilker and Meiners 2002, 2006; Turlings and Wäckers 2004; Dicke 2009; Dicke et al. 2009). In cases known thus far, the plant’s response is triggered by compounds present either in the regurgitant or in the egg-associated secretion of the herbivore (Mattiacci et al. 1995; Alborn et al. 1997; Halitschke et al. 2001; Colazza et al. 2004a; Hilker et al. 2005; Felton and Tumlinson 2008). Brussels sprouts plants respond to P. brassicae eggs by modifying the leaf surface, which arrests T. brassicae wasps three days after egg deposition when the host eggs are most suitable for parasitism (Fatouros et al. 2005b). The leaf surface changes could be of a chemical nature, but remain unknown. However, genome-wide transcriptional analysis has provided molecular evidence that confirms oviposition- and BC-induced changes in the plant (Fatouros et al. 2008a). In female P. brassicae, traces of BC (the male anti-aphrodisiac) were detected in the secretion from the accessory reproductive gland (ARG), which is released with eggs onto the plant surface (Fatouros et al. 2008a).
Whereas P. brassicae deposits egg clutches consisting of 20–50 eggs (Feltwell 1982), the closely related small cabbage white, P. rapae L., lays single eggs on wild and cultivated Brassicaceae (Richards 1940). Male P. rapae butterflies transfer methyl salicylate (MeSA) and indole with their ejaculate to the females. Mated P. rapae females emitting MeSA and indole were unattractive to conspecific males, thus showing that both compounds function as an anti-aphrodisiac blend (Andersson et al. 2003). The aim of this study was to investigate whether singly laid eggs of P. rapae induce the production of a plant synomone in Brussels sprouts that arrests T. brassicae wasps, and, if so, whether this plant response is triggered by male-derived compounds transferred within the ejaculate during mating, specifically, the anti-aphrodisiac compounds indole and/or methyl salicylate.
Methods and Materials
Plants and Insects
Brussels sprouts plants (Brassica oleracea L. var. gemmifera cv. Cyrus) were grown in a greenhouse (18 ± 5°C, 50–70% rh, L16:D8). Pieris rapae was reared on Brussels sprouts in a climate room (21 ± 1°C, 50–70% rh, L16:D8). Virgin females were obtained by separating sexes in the pupal phase, and keeping them separately from males after eclosion until dissection. Three days after eclosion, the ARGs were dissected from females. Mated females were obtained by adding a virgin male to a virgin female one day after eclosion. As soon as a butterfly couple was observed to mate, it was isolated in a separate cage to obtain females for dissection that had mated for the first time. Two days after mating, the ARG were dissected from females.
Trichogramma brassicae (Hymenoptera: Trichogrammatidae) was reared in irradiated eggs of the moth Ephestia kuehniella Zeller (Lepidoptera: Pyralidae), received from Koppert B.V., The Netherlands, at 25 ± 1°C, 50–70% RH, L16:D8 in a climate chamber. Ephestia kuehniella eggs were glued on paper cards and offered to the wasps in glass vials for parasitization. Trichogramma wasps are known to be capable of developing successfully in Ephestia eggs (Brower 1983). Only mated, 2-5-d-old, oviposition-experienced female wasps were used for the experiments. An oviposition experience was given for a period of 18 h prior to the experiment with ≤3-d-old P. rapae eggs deposited on Brussels sprouts leaves. Eggs older than 3 d are unsuitable for parasitization because of sclerotization of the caterpillar’s head capsule.
Preparation of ARG Homogenates
To obtain samples for bioassays, ARGs were dissected from 3 gravid or virgin P. rapae females (3-d-old) in phosphate buffered saline (PBS; pH 7.2), transferred to a vial with 100 μl PBS, and homogenized. Then, after addition of another 100 μl of PBS, the homogenate was centrifuged, and 100 μl of the supernatant were applied with a brush to the edge of a cabbage leaf as described below (an equivalent of 1.5 extracted glands per leaf). For chemical analysis, 1 ARG of either a mated or a virgin P. rapae female (3-d-old) was dissected and transferred to a vial containing 50 μl of dichloromethane (DCM) with ethyl salicylate (EtSA) as internal standard (0.25 ng/µl). In total, 7 samples of each gland type were analyzed by GC-MS.
Plant Treatments
For bioassays with egg-infested plants, test plants were placed into a cage with more than 100 P. rapae adults to allow deposition of eggs, wing scales, and host odors onto the plants. Plants were exposed for about 1–2 h to the butterflies, with a maximum of 20 eggs deposited per leaf. Up to 20 P. rapae eggs have been observed on a single Brassica oleracea plant in nature (N.E. Fatouros, personal observations). After this exposure time, the egg-infested plants were tested immediately or were kept in a climate chamber (21 ± 2°C, 70% rh, L16:D8) either overnight (24 h) or 48 to 72 h after the day of egg deposition. Thus, the period during which eggs or butterfly deposits could affect the cabbage plant was in total 6, 24, 48, 72, or 96 h. Control plants were grown under the same conditions as treated plants, but were never in contact with P. rapae or other insects.
For bioassays with ARG homogenate-treated plants, a sample of ARG homogenate of mated P. rapae females was applied to the edge of a Brussels sprouts leaf in a stretch of about 2 cm on the abaxial leaf side. As a control, a sample of ARG homogenate of virgin P. rapae females was applied to the plants in the same way. After treatment, all plants were kept for either 24 or 72 h in a climate chamber (21 ± 1°C, 50–70% RH, L16:D8).
For bioassays with ARG homogenate and indole treated plants, 30 μl of methanolic indole solution (10 ng indole (Sigma-Aldrich) in 100 μl MeOH) were added to 170 μl of ARG homogenate from virgin females, and 100 μl of this mixture were applied to a leaf as above (i.e., 1.5 ng indole, plus ARG homogenate from virgin females equivalents 1.5 ARGs from mated females). Test leaf squares were cut from the plant 72 h after application of the mixture. Control leaf squares were obtained from leaves treated with ARG homogenate of virgin females in PBS and MeOH.
For bioassays with indole treated plants, 100 μl of 0.005 or 0.5 ng indole/μl MeOH solutions were applied to leaves as described above. Control plants were treated with 100 μl MeOH only. Leaf squares adjacent to the indole treatment were tested against leaf squares adjacent to the solvent treatment in a two-choice bioassay.
Two-chamber Olfactometer Bioassays
The experiments were carried out in a two-chamber olfactometer described in detail by Fatouros et al. (2005a). Time spent by wasps in one of the two odor fields was measured for 300 s. The 4th or 5th leaf from the top of a plant was excised, and kept with its petiole in a vial with water during the bioassay. A total of 10 T. brassicae wasps were tested per day per plant. In total, 50 wasps per treatment were tested, and 5 plants per treatment were used. To avoid biased results due to positional preferences of the parasitoids, the olfactometer was rotated 180˚ after every third insect tested. The response of T. brassicae was tested to odors of plants infested with P. rapae eggs in the following two-choice combinations: a) plants with eggs 24 h after deposition vs. clean air and, b) plants with eggs 72 h after egg deposition vs. clean air. All two-chamber olfactometer bioassays were statistically analyzed using Wilcoxon matched pairs test by using SPSS for Windows 15.0 Software.
Two-choice Contact Bioassays
All contact bioassays were conducted with egg-free or untreated leaf squares (in Fatouros et al. 2005a, 2008a, denoted as ‘locally induced’) cut close to a treated leaf part, i.e., single eggs, applied ARG homogenate and/or indole/MeOH solution. A female wasp was released in a glass Petri dish (5.5 cm diam) halfway between a test, and control leaf squares (1.5 cm2) directly cut from the plants immediately prior to the bioassay. The total time spent (i.e., residence time) on each of the leaf squares was observed for a period of 300 s by using The Observer software v. 4.0 (Noldus Information Technology, Wageningen, The Netherlands). A detailed description of the bioassay method is given elsewhere (Fatouros et al. 2005a). Test and control squares were taken from leaves of corresponding size and position on the plants. In total, 50 wasps per treatment were tested, and 5 plants per treatment were used. A maximum of 10 wasps per experimental day were tested. Leaf squares were renewed and repositioned randomly after every third wasp tested. All two-choice contact bioassays were analyzed using Wilcoxon matched pairs test by using SPSS 15.0 for Windows Software.
Chemical Analysis
ARG extracts were analyzed by coupled gas chromatography—mass spectrometry (GC-MS) using a gas chromatograph (Agilent 7890A) equipped with a 30-m Zebron ZB-5 ms column (0.25 mm i.d., 0.25-μm film thickness; Phenomenex, Torrance, CA, USA) and an inert mass selective detector (model 5975C with triple axis detector, Agilent). A 5 m Guardian™ pre-column (deactivated fused silica tubing without stationary phase; Phenomenex, Torrance, USA), was permanently attached to the analytical column. The GC was programmed from 45°C for 1 min, to 200°C at 10°C min−1, then to 280°C at 30°C min−1, and held 3.5 min. The sample volume (1 μl) was injected in splitless mode. The injection port and interface temperature were 250°C and 280°C, respectively, and the helium inlet pressure was controlled electronically to achieve a constant column flow of 1.0 ml min−1. A solvent delay was set to 4 min. The ionization potential was set at 70 eV, and scanning was performed from 33 to 200 atomic mass units. Identification of MeSA, indole and the internal standard EtSA was based on the injection of authentic reference standards (>98% purity, Sigma-Aldrich). Quantification of MeSA and indole was based on comparison with the internal standard. A calibration series of MeSA, indole, and EtSA, injected from a concentration of 0.05–50 ng/μl DCM, showed similar linear response factors of all three compounds within this concentration range (data not shown). Differences in quantities of anti-aphrodisiac compounds were analyzed using Mann-Whitney U test by using SPSS 15.0 for Windows Software.
Results
Olfactory Response of Trichogramma to Leaves Infested with P. rapae Eggs
Trichogramma brassicae did not discriminate between volatiles from an egg-laden leaf and clean air (24 h: P = 0.426, 72 h: P = 0.903, Wilcoxon matched pairs test).
Arrestment of Trichogramma by Leaves Infested with P. rapae Eggs
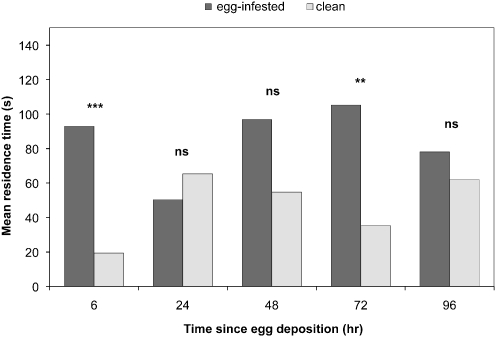
Leaf squares from plants, on which eggs had been deposited 6 h prior to the assay arrested T. brassicae when tested vs. leaf squares from uninfested plants (Fig. 1, P < 0.001, Wilcoxon matched pairs test). However, leaf squares from plants on which eggs had been deposited 24 h prior to the assay did not arrest the wasps (Fig. 1, P = 0.494, Wilcoxon matched pairs test). The wasps tended to prefer leaf squares cut from an egg-carrying leaf on which eggs had been deposited 48 h prior to the assay when tested vs. leaf squares from uninfested plants (Fig. 1a, P = 0.058, Wilcoxon matched pairs test). Wasps stayed longer on leaf squares of plants with eggs deposited 72 h prior to the bioassay (Fig. 1, P = 0.004, Wilcoxon matched pairs test). Four days after egg deposition, wasps did not discriminate between leaf squares of egg-infested plants when tested vs. leaf squares of uninfested plants (Fig. 1a, P = 0.427, Wilcoxon-matched pairs test).
Fig. 1.
Mean residence time of Trichogramma brassicae females on Brussels sprouts leaves infested with Pieris rapae eggs (egg-infested) tested against leaves of uninfested plants (clean). Egg-free leaf squares adjacent to eggs that had been deposited 6-96 h prior to the bioassay (dark grey columns) were simultaneously offered against leaf squares from uninfested plants (light grey columns) in a two-choice contact bioassay in a Petri dish. Number of tested females per treatment, N = 50. Abbreviations: ns—not significant; **P < 0.01; ***P < 0.001 (Wilcoxon matched pairs test)
Arrestment of Trichogramma by Leaves Treated with ARG
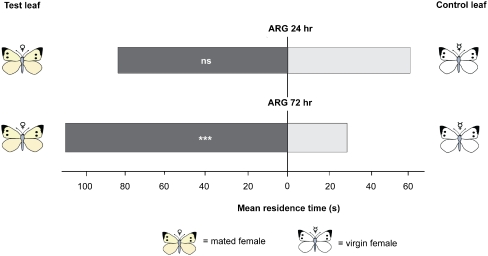
Untreated leaf squares obtained from leaves treated with ARG homogenates of mated females were tested against untreated leaf squares from leaves treated with ARG homogenate of virgin females 24 h after treatment. The wasps did not discriminate between the two leaf squares cut from the vicinity of leaf area treated with the ARG homogenate (Fig. 2, P = 0.184, Wilcoxon matched pairs test). However, 72 h after ARG application, the wasps spent significantly more time on leaf squares taken from plants treated with ARG extract of mated P. rapae females than on leaf squares from plants treated with ARG extract of virgin P. rapae females (Fig. 2, P < 0.001, Wilcoxon matched pairs test).
Fig. 2.
Mean residence time of Trichogramma brassicae females on Brussels sprouts leaves treated with accessory reproductive gland (ARG) homogenate of Pieris rapae females. Leaves were treated either with ARG homogenate from virgin females (light grey bars) or ARG homogenate from mated females (dark grey bars) a) 24 h or b) 72 h prior to the bioassay. Untreated leaf squares, adjacent to a site on the same leaf that was treated with ARG homogenate, were simultaneously offered to the wasps in a two-choice contact bioassay in a Petri dish. Number of tested females wasps per bioassay combination, N = 50. Abbreviations: ARG—accessory reproductive gland; ns—not significant; ***P < 0.001 (Wilcoxon matched pairs test)
Chemical Composition of ARG Extracts
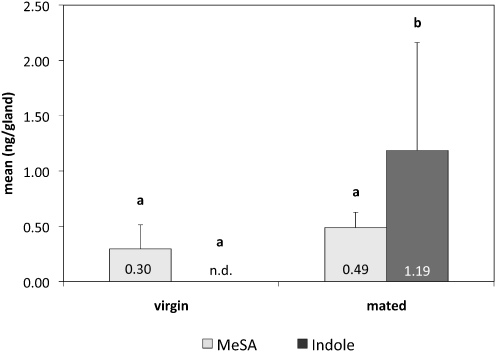
Chemical analysis of ARG secretion of mated females revealed the presence of the anti-aphrodisiac compounds (Andersson et al. 2003), indole and MeSA. In ARG extracts obtained from females 2 days after mating, 1.2 ± 0.97 ng indole and 0.49 ± 0.14 MeSA were detected (Fig. 3). In ARG extracts of virgin females, 0.30 ± 0.22 ng MeSA / ARG was detected, whereas indole was lacking (Fig. 3, P < 0.001, Mann-Whitney U test).
Fig. 3.
Quantities of anti-aphrodisiac compounds detected by GC-MS in accessory reproductive gland (ARG) extracts from virgin or mated Pieris rapae females. Mean (± s.d.) amount of methyl salicylate (grey columns) and indole (dark grey column) per gland is shown. Number of dissected ARG, N = 7 of each treatment. Different letters indicate significant differences in quantity within the same compound between the ARG extracts from virgin and mated females (Mann-Whitney U test). Abbreviations: ARG—accessory reproductive gland; MeSA—methyl salicylate; n.d.—not detected
Arrestment of Trichogramma by Leaves Treated with Indole
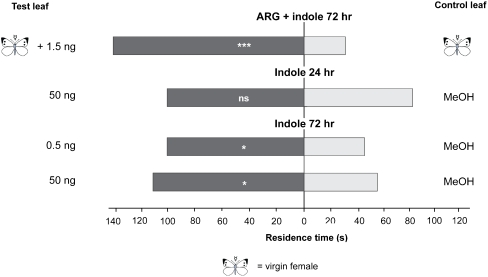
Untreated leaf squares taken from leaves treated with ARG homogenate from virgin females supplemented with 1.5 ng indole arrested the wasps 72 h after application when tested against untreated leaf squares obtained from leaves treated only with ARG homogenate from virgin females (Fig. 4, P < 0.001, Wilcoxon matched pairs test). Leaf squares of plants treated with 50 ng indole alone did not arrest the wasps 24 h after treatment (Fig. 4, P = 0.134, Wilcoxon matched pairs test). Leaf squares of leaves treated with indole 72 h after treatment arrested T. brassicae wasps when tested against leaf squares of plants treated with solvent only at both tested concentrations (Fig. 4, 0.5 ng: P = 0.028; 50 ng: P = 0.036, Wilcoxon matched pairs test).
Fig. 4.
Mean residence time of Trichogramma brassicae females on Brussels sprouts leaves treated with Accessory Reproductive Gland (ARG) homogenate of virgin females and indole or treated with indole alone (amount shown in ng). Test leaves were treated either with ARG extracts from virgin P. rapae females and indole or indole alone (dark grey bars) and tested against control leaves treated with ARG extracts from virgin Pieris rapae females or methanol only (light grey bars) a) 24 h or b) 72 h prior to the bioassay. Untreated leaf area, adjacent to a site on the same leaf that was treated with ARG homogenate and/or indole was simultaneously offered against leaf squares taken from ARG-homogenate or methanol-treated leaves in a two-choice contact bioassay in a Petri dish. Female wasps per bioassay combination, N = 50. Abbreviations: MeOH—methanol; ARG—accessory reproductive gland; ns—not significant; *P < 0.05; ***P < 0.001 (Wilcoxon matched pairs test)
Discussion
Our data show that egg deposition of the small cabbage white butterfly, P. rapae, induces Brussels sprouts plants such that T. brassicae wasps are arrested 72 h after oviposition, whereas plants with eggs deposited 24 or 48 h before testing did not elicit this behavior (Fig. 1). Thus, we demonstrated that butterfly eggs deposited singly and spaced can trigger an indirect plant response similar to that induced by egg batches of herbivorous insects (Meiners and Hilker 2000; Hilker et al. 2002; Colazza et al. 2004a; Fatouros et al. 2005a). Most butterfly species deposit their eggs singly, and these often are cryptically colored. It is assumed that natural selection by predators and parasitoids favors this strategy. Singly laid eggs probably have lower amounts of toxins relative to the often aposematically colored eggs laid in clutches (Stamp 1980). We expected that the induced response of Brussels sprouts plants to eggs of P. rapae would be less detectable by Trichogramma wasps than the response to P. brassicae eggs. This contingency would have been consistent with the findings of Little et al. (2007) who showed that deposition of P. rapae eggs on Arabidopsis thaliana caused a much weaker transcriptional response of defense- and stress-related genes than did P. brassicae egg clutches.
When an extract from accessory reproductive glands (ARGs) of mated female butterflies was applied to plants, the wasps were arrested on treated plants 72 h after ARG treatment, but not after 24 h. This shows that the ARG secretion itself triggered the plant response and not the eggs per se. One known function of the ARG is the secretion of adhesive polymers to attach eggs onto the leaf surface (Gillott 2002). The ARG extract from virgin females applied onto the plant arrested the wasps neither 24 nor 72 h after application (Fig. 2). Evidently, compounds derived from the seminal fluid of the male butterfly have changed the composition of the female’s ARG, ultimately playing a part in triggering the plant response.
Our evidence indicates that the male anti-aphrodisiac compound, indole, reaches the ARG of a mated P. rapae female. No indole was found in the ARG of virgin females (Fig. 3). Subsequently, T. brassicae wasps had a significant preference for leaves treated with the ARG of virgin females plus indole over leaves treated with ARG extract from virgin females. The parasitoids’ response to leaves treated with indole alone (without ARG extract) 72 h after application was lower but still significant (Fig. 4). Thus, a single compound, which is part of the two-component anti-aphrodisiac blend of P. rapae males and transferred with the male ejaculate, is likely to trigger this plant defense in response to egg deposition. Earlier, Doss et al. (2000) showed that esters of long-chain diols (the so-called bruchins) elicit formation of callus tissue in pea pods when certain species of Bruchidae (Coleoptera) oviposit in pods. Our discoveries that anti-aphrodisiac compounds in Pieris spp. act similarly as elicitors add to the known instance of this type of effect (Fatouros et al. 2008a).
Interestingly, the anti-aphrodisiac compounds of Pieris spp. (BC, MeSA and indole) are ubiquitous phytochemicals produced, for example, as part of herbivore-induced plant volatiles (HIPV) from several plant species; e.g., Lima bean (Dicke et al. 1990; De Boer et al. 2004), tomato (Ament et al. 2004), and maize (D'Alessandro and Turlings 2005). Cultivated Brassica species and Arabidopsis thaliana emit BC, MeSA, and indole after feeding by Pieris caterpillars or after the application of their regurgitant (Geervliet et al. 1997; van Poecke et al. 2001; Smid et al. 2002; Fatouros et al. 2005c). The biological relevance of these HIPV components has been proven for many herbivorous and carnivorous species. Methyl salicylate and indole derivates repel parasitoids of lepidopteran larvae (D'Alessandro et al. 2006; Snoeren 2009) and ovipositing lepidopterans (De Vos et al. 2008; Ulland et al. 2008), whereas predatory arthropods are attracted (De Boer and Dicke 2004; De Boer et al. 2004; James and Price 2004; Ishiwari et al. 2007). In several plant species, the two aromatic compounds, MeSA and indole, are formed via the shikimic acid pathway (Paré and Tumlinson 1997), whereas the nitrile (BC) is a myrosinase-catalyzed hydrolysis product of glucosinolates in Brassicaceae (reviewed by Grubb and Abel 2006; Hopkins et al. 2009). However, in Pieris, BC, MeSA, and indole are not plant-derived; rather, males utilize the amino acids phenylalanine and tryptophan as precursors for these anti-aphrodisiac compounds (Andersson et al. 2000, 2003).
The waxy leaf surface of Brussels sprouts plants shows no apparent damage below or around eggs of Pieris spp. (see Chapman and Bernays 1989 and references therein; N. E. Fatouros, personal observations). All other studies of indirect plant responses against herbivore oviposition involved wounding by the ovipositing female, leading to emission of volatiles attractive to egg parasitoids (Hilker and Meiners 2006). For examples, eggs of an elm leaf beetle (Xanthogaleruca luteola) and a pine sawfly (Diprion pini) are laid on the plant surface or into tissue damaged by the egg-laying female prior to oviposition; the oviposition-associated leaf damage in these species may provide improved attachment to the plant or protection of the eggs by the plant tissue (Meiners and Hilker 1997, 2000; Hilker et al. 2002). Eggs laid by the heteropteran, Nezara viridula, induced a volatile synomone for the egg parasitoid Trissolcus basalis in two legume species (Vicia faba and Phaseolus vulgaris), but only when oviposition and host feeding occurred together on the same plant (Colazza et al. 2004a, 2004b). Thus, we assumed that wounding of the leaf surface during or immediately preceding the egg deposition is required to induce volatile long-range cues that attract the egg parasitoids (Fatouros et al. 2005b). Yet, evidence is accumulating that in other Brassica species that carry less surface waxes, Pieris egg deposition induces volatile emissions attractive to Trichogramma wasps (N. E. Fatouros, unpublished data). Differences in the physical structure and/or chemical composition of the plant cuticle could play an important role in the adsorption and/or absorption of molecules into leaf cells, thus triggering certain pathways involved in plant defense against herbivores. Leaf wax microstructure and chemical composition affect herbivores, and also indirectly influence their predators and parasitoids (Eigenbrode and Espelie 1995). For example, an increased surface wax layer has been found to confer higher resistance to herbivores (see Müller and Riederer 2005 and references therein).
Egg-free leaf parts of plants on which eggs had been deposited elsewhere 6 h before testing did arrest T. brassicae wasps. This arrestment is probably due to host residues, such as wing scales, as Fatouros et al. (2005a) showed that T. brassicae responded to P. brassicae egg-free leaves contaminated with butterfly deposits for 24 h; however, the response disappeared after 72 h. Scales and other chemical traces of host insects frequently are used as kairomones by egg parasitoids (reviewed by Fatouros et al. 2008b). The wings of Pieris butterflies emit chemical cues, some of which have pheromonal activity (Arsene et al. 2002; Andersson et al. 2007). The aphrodisiacs from male scent scales of P. rapae and P. brassicae (two macrolides) recently have been identified (Yildizhan et al. 2009). Whether these macrolides arrest wasps on leaves with eggs younger than 24 h remains to be tested.
Sexual signals produced that attract mates (or that repel the competing sex as in the case of anti-aphrodisiac pheromones) potentially incur trade-offs associated with their use as kairomones by natural enemies (Stowe et al. 1995; Zuk and Kolluru 1998; Fatouros et al. 2008b . It is assumed that female sex pheromones are rarely exploited because of their high specificity, low intensity, and emission in enemy-free space and time (Zuk and Kolluru 1998). However, an increasing number of studies have shown that egg parasitoids in particular exploit adult host sex pheromones (Fatouros et al. 2008b). In contrast to sex pheromones, the release of anti-aphrodisiacs is unlikely to be under female control (Andersson et al. 2004). There should be enough time for egg parasitoids to detect and approach a mated female, in contrast to a virgin, to successfully parasitize fertilized host eggs. By triggering parasitoid-attracting plant cues, the anti-aphrodisiac compounds incur an additional cost of mating, namely, increased egg mortality and lower butterfly fitness. We postulate, therefore, that the evolution of anti-aphrodisiac compounds transferred during copulation by the males is selected against by Trichogramma wasps.
Acknowledgements
The authors thank Jhamna Castillo Solera for conducting preliminary tests, Leo Koopman, Frans van Aggelen, André Gidding, and Léon Westerd for rearing the butterflies, and the experimental farm of Wageningen University (Unifarm) for growing Brussels sprouts plants. The authors acknowledge funding from German Research Foundation grants DFG Hi 416/15-1,2 (to M.H.) and FA 824/1-11 (to N.E.F.), the Netherlands Organization for Scientific Research NWO/ALW VENI grant 863.05.020 (to M.E.H.) and NWO/ALW VICI 865.03.002 (to R.M. and M.D.), the European Science Foundation (ESF) for a Two-Month Exchange Grant (to N.E.F.) and the United States Department of Education P116J03019 and Alfred P. Sloan Foundation Graduate Scholarship (to W.V.A.C.).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949. doi: 10.1126/science.276.5314.945. [DOI] [Google Scholar]
- Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 2004;135:2025–2037. doi: 10.1104/pp.104.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J, Borg-Karlson A-K, Wiklund C. Sexual cooperation and conflict in butterflies: a male-transferred anti-aphrodisiac reduces harassment of recently mated females. Proc. Roy. Soc. Lond. B. 2000;267:1271–1275. doi: 10.1098/rspb.2000.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J, Borg-Karlson A-K, Wiklund C. Antiaphrodisiacs in pierid butterflies: a theme with variation! J. Chem. Ecol. 2003;29:1489–1499. doi: 10.1023/A:1024277823101. [DOI] [PubMed] [Google Scholar]
- Andersson, J., Borg-Karlson, A.-K., and Wiklund, C. 2004. Sexual conflict and anti-aphrodisiac titre in a polyandrous butterfly: male ejaculate tailoring and absence of female control. Proc. Roy. Soc. Lond. B 271:1765–1770. [DOI] [PMC free article] [PubMed]
- Andersson J, Borg-Karlson A-K, Vongvanich N, Wiklund C. Male sex pheromone release and female mate choice in a butterfly. J. Exp. Biol. 2007;210:964–970. doi: 10.1242/jeb.02726. [DOI] [PubMed] [Google Scholar]
- Arsene C, Schulz S, Van Loon JJA. Chemical polymorphism of the cuticular lipids of the cabbage white Pieris rapae. J. Chem. Ecol. 2002;28:2627–2631. doi: 10.1023/A:1021474820601. [DOI] [PubMed] [Google Scholar]
- Boggs CL. A General model of the role of male-donated nutrients in female insects’ reproduction. Am. Nat. 1990;136:598. doi: 10.1086/285118. [DOI] [Google Scholar]
- Boggs CL, Gilbert LE. Male contribution to egg production in butterflies: evidence for transfer of nutrients at mating. Science. 1979;206:83–84. doi: 10.1126/science.206.4414.83. [DOI] [PubMed] [Google Scholar]
- Brower J. Eggs of stored-product lepidoptera as hosts for Trichogramma evanescens [Hym.: Trichogrammatidae] BioControl. 1983;28:355–361. [Google Scholar]
- Chapman RF, Bernays EA. Insect behavior at the leaf surface and learning as aspects of host plant selection. Experienta. 1989;45:215–222. doi: 10.1007/BF01951806. [DOI] [Google Scholar]
- Colazza S, Fucarino A, Peri E, Salerno G, Conti E, Bin F. Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J. Exp. Biol. 2004;207:47–53. doi: 10.1242/jeb.00732. [DOI] [PubMed] [Google Scholar]
- Colazza S, Mcelfresh JS, Millar JG. Identification of volatile synomones, induced by Nezara viridula feeding and oviposition on bean spp., that attract the egg parasitoid Trissolcus basalis. J. Chem. Ecol. 2004;30:945–964. doi: 10.1023/B:JOEC.0000028460.70584.d1. [DOI] [PubMed] [Google Scholar]
- D'Alessandro M, Turlings TCJ. In situ modification of herbivore-induced plant odors: a novel approach to study the attractiveness of volatile organic compounds to parasitic wasps. Chem. Senses. 2005;30:739–753. doi: 10.1093/chemse/bji066. [DOI] [PubMed] [Google Scholar]
- D'Alessandro M, Held M, Triponez Y, Turlings TCJ. The role of indole and other shikimic acid derived maize volatiles in the attraction of two parasitic wasps. J. Chem. Ecol. 2006;32:2733–2748. doi: 10.1007/s10886-006-9196-7. [DOI] [PubMed] [Google Scholar]
- De Boer JG, Dicke M. The role of methyl salicylate in prey searching behavior of the predatory mite Phytoseiulus persimilis. J. Chem. Ecol. 2004;30:255–271. doi: 10.1023/B:JOEC.0000017976.60630.8c. [DOI] [PubMed] [Google Scholar]
- De Boer JG, Posthumus MA, Dicke M. Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. J. Chem. Ecol. 2004;30:2215–2230. doi: 10.1023/B:JOEC.0000048784.79031.5e. [DOI] [PubMed] [Google Scholar]
- De Vos M, Kriksunov KL, Jander G. Indole-3-acetonitrile production from indole glucosinolates deters oviposition by Pieris rapae. Plant Physiol. 2008;146:916–926. doi: 10.1104/pp.107.112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicke M. Behavioural and community ecology of plants that cry for help. Plant Cell Envir. 2009;32:654–665. doi: 10.1111/j.1365-3040.2008.01913.x. [DOI] [PubMed] [Google Scholar]
- Dicke M, Van Beek TA, Posthumus MA, Ben Dom N, Van Bokhoven H, De Groot A. Isolation and identification of volatile kairomone that affects acarine predatorprey interactions involvement of host plant in its production. J. Chem. Ecol. 1990;16:381–396. doi: 10.1007/BF01021772. [DOI] [PubMed] [Google Scholar]
- Dicke M, Van Loon JJA, Soler R. Chemical complexity of volatiles from plants induced by multiple attack. Nat. Chem. Biol. 2009;5:317–324. doi: 10.1038/nchembio.169. [DOI] [PubMed] [Google Scholar]
- Doss RP, Oliver JE, Proebsting WM, Potter SW, Kuy SR, Clement SL, Williamson RT, Carney JR, Devilbiss ED. Bruchins: Insect–derived plant regulators that stimulate neoplasm formation. Proc. Natl. Acad. Sci. USA. 2000;97:6218–6223. doi: 10.1073/pnas.110054697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenbrode SD, Espelie KE. Effects of plant epicuticular lipids on insect herbivores. Annu. Rev. Entomol. 1995;40:171–194. doi: 10.1146/annurev.en.40.010195.001131. [DOI] [Google Scholar]
- Fatouros NE, Bukovinszkine'kiss G, Kalkers LA, Soler Gamborena R, Dicke M, Hilker M. Oviposition-induced plant cues: do they arrest Trichogramma wasps during host location? Entomol. Exp. Appl. 2005;115:207–215. doi: 10.1111/j.1570-7458.2005.00245.x. [DOI] [Google Scholar]
- Fatouros NE, Huigens ME, Van Loon JJA, Dicke M, Hilker M. Chemical communication—butterfly anti-aphrodisiac lures parasitic wasps. Nature. 2005;433:704. doi: 10.1038/433704a. [DOI] [PubMed] [Google Scholar]
- Fatouros NE, Van Loon JJA, Hordijk KA, Smid HM, Dicke M. Herbivore-induced plant volatiles mediate in-flight host discrimination by parasitoids. J. Chem. Ecol. 2005;31:2033–2047. doi: 10.1007/s10886-005-6076-5. [DOI] [PubMed] [Google Scholar]
- Fatouros NE, Broekgaarden C, Bukovinszkine'kiss G, Van Loon JJA, Mumm R, Huigens ME, Dicke M, Hilker M. Male-derived butterfly anti-aphrodisiac mediates induced indirect plant defense. Proc. Natl. Acad. Sci. USA. 2008;105:10033–10038. doi: 10.1073/pnas.0707809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouros NE, Dicke M, Mumm R, Meiners T, Hilker M. Foraging behavior of egg parasitoids exploiting chemical information. Behav. Ecol. 2008;19:677–689. doi: 10.1093/beheco/arn011. [DOI] [Google Scholar]
- Felton GW, Tumlinson JH. Plant-insect dialogs: complex interactions at the plant-insect interface. Curr. Biol. 2008;11:457–463. doi: 10.1016/j.pbi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Feltwell J. Large white butterfly—the biology, biochemistry and physiology of Pieris brassicae (Linnaeus) The Hague-Boston-London: Dr. W. Junk Publishers; 1982. [Google Scholar]
- Geervliet JBF, Posthumus MA, Vet LEM, Dicke M. Comparative analysis of headspace volatiles from different caterpillar-infested or uninfested food plants of Pieris species. J. Chem. Ecol. 1997;23:2935–2954. doi: 10.1023/A:1022583515142. [DOI] [Google Scholar]
- Gillott C. Insect accessory reproductive glands: key players in production and protection of eggs. In: Hilker M, Meiners T, editors. Chemoecology of insect eggs and egg deposition. Berlin, Oxford: Blackwell; 2002. pp. 37–59. [Google Scholar]
- Grubb CD, Abel S. Glucosinolate metabolism and its control. Trends Plant Sci. 2006;11:89–100. doi: 10.1016/j.tplants.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Gwynne DT. Sexual conflict over nuptial gifts in insects. Annu. Rev. Entomol. 2008;53:83–101. doi: 10.1146/annurev.ento.53.103106.093423. [DOI] [PubMed] [Google Scholar]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Spingidae) and its natural host Nicotinia attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001;125:711–717. doi: 10.1104/pp.125.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilker M, Meiners T. Induction of plant responses to oviposition and feeding by herbivorous arthropods: a comparison. Entomol. Exp. Appl. 2002;104:181–192. doi: 10.1023/A:1021232319226. [DOI] [Google Scholar]
- Hilker M, Meiners T. Early herbivore alert: insect eggs induce plant defense. J. Chem. Ecol. 2006;32:1379–1397. doi: 10.1007/s10886-006-9057-4. [DOI] [PubMed] [Google Scholar]
- Hilker M, Kobs C, Varama M, Schrank K. Insect egg deposition induces Pinus sylvestris to attract egg parasitoids. J. Exp. Biol. 2002;205:455–461. doi: 10.1242/jeb.205.4.455. [DOI] [PubMed] [Google Scholar]
- Hilker M, Stein C, Schröder R, Varama M, Mumm R. Insect egg deposition induces defence responses in Pinus sylvestris: Characterisation of the elicitor. J. Exp. Biol. 2005;208:1849–1854. doi: 10.1242/jeb.01578. [DOI] [PubMed] [Google Scholar]
- Hopkins RJ, Van Dam NM, Van Loon JJA. Role of Glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009;54:57–83. doi: 10.1146/annurev.ento.54.110807.090623. [DOI] [PubMed] [Google Scholar]
- Huigens ME, Pashalidou FG, Qian M-H, Bukovinszky T, Smid HM, Van Loon JJA, Dicke M, Fatouros NE. Hitch-hiking parasitic wasp learns to exploit butterfly antiaphrodisiac. Proc. Natl. Acad. Sci. USA. 2009;106:820–825. doi: 10.1073/pnas.0812277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwari H, Suzuki T, Maeda T. Essential compounds in herbivore-induced plant volatiles that attract the predatory mite Neoseiulus womersleyi. J. Chem. Ecol. 2007;33:1670–1681. doi: 10.1007/s10886-007-9344-8. [DOI] [PubMed] [Google Scholar]
- James DG, Price TS. Field-testing of methyl salicylate for recruitment and retention of beneficial insects in grapes and hops. J. Chem. Ecol. 2004;30:1613–1628. doi: 10.1023/B:JOEC.0000042072.18151.6f. [DOI] [PubMed] [Google Scholar]
- Little D, Gouhier-Darimont C, Bruessow F, Reymond P. Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant Physiol. 2007;143:784–800. doi: 10.1104/pp.106.090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiacci L, Dicke M, Posthumus MA. β-Glucosidase: an elicitor of the herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc. Natl. Acad. Sci. USA. 1995;92:2036–2040. doi: 10.1073/pnas.92.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiners T, Hilker M. Host location in Oomyzus gallerucae (Hymenoptera: Eulophidae), an egg parasitoid of the elm leaf beetle Xanthogaleruca luteola (Coleoptera: Chrysomelidae) Oecologia. 1997;112:87–93. doi: 10.1007/s004420050287. [DOI] [PubMed] [Google Scholar]
- Meiners T, Hilker M. Induction of plant synomones by oviposition of a phytophagous insect. J. Chem. Ecol. 2000;26:221–232. doi: 10.1023/A:1005453830961. [DOI] [Google Scholar]
- Müller C, Riederer M. Plant surface properties in chemical ecology. J. Chem. Ecol. 2005;31:2621–2651. doi: 10.1007/s10886-005-7617-7. [DOI] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH. Induced synthesis of plant volatiles. Nature. 1997;385:30–31. doi: 10.1038/385030a0. [DOI] [Google Scholar]
- Powell W. Parasitoid hosts. In: Hardie J, Minks AK, editors. Pheromones of Non-Lepidopteran Insects Associated with Agricultural Plants. Wallingford: CABI; 1999. pp. 405–427. [Google Scholar]
- Richards OW. The biology of the small white butterfly (Pieris rapae), with special reference to the factors controlling its abundance. J. Animal Ecol. 1940;9:243–288. doi: 10.2307/1459. [DOI] [Google Scholar]
- Smid HM, Van Loon JJA, Posthumus MA, Vet LEM. GC-EAG-analysis of volatiles from Brussels sprouts plants damaged by two species of Pieris caterpillars: Olfactory receptive range of a specialist and a generalist parasitoid wasp species. Chemoecology. 2002;12:169–176. doi: 10.1007/PL00012665. [DOI] [Google Scholar]
- Snoeren, T. 2009. Herbivore-induced indirect defense in Arabidopsis. Ecogenomic approach to the role of infochemicals in parasitoid attraction. Ph.D. Wageningen University. Wageningen.
- Stamp NE. Egg deposition patterns in butterflies: why do some species cluster their eggs rather than deposit them singly? Am. Nat. 1980;115:367–380. doi: 10.1086/283567. [DOI] [Google Scholar]
- Stowe MK, Turlings TCJ, Loughrin JH, Lewis WJ, Tumlinson JH. The chemistry of eavesdropping, alarm, and deceit. Proc. Natl. Acad. Sci. USA. 1995;92:23–28. doi: 10.1073/pnas.92.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlings TCJ, Wäckers F. Recruitment of predators and parasitoids by herbivore-injured plants. In: Carde R, Millar JG, editors. Advances in Insect Chemical Ecology. Cambridge: Cambridge University Press; 2004. pp. 21–75. [Google Scholar]
- Ulland S, Ian E, Stranden M, Borg-Karlson A-K, Mustaparta H. Plant volatiles activating specific olfactory receptor neurons of the cabbage moth Mamestra brassicae L. (Lepidoptera, Noctuidae). Chem. Senses. 2008;33:509–522. doi: 10.1093/chemse/bjn018. [DOI] [PubMed] [Google Scholar]
- Van Poecke RMP, Posthumus MA, Dicke M. Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: chemical, behavioral, and gene-expression analysis. J. Chem. Ecol. 2001;27:1911–1928. doi: 10.1023/A:1012213116515. [DOI] [PubMed] [Google Scholar]
- Vinson SB. How parasitoids locate their hosts: a case of insect espionage. In: Lewis T, editor. Insect Communication. London: Academic; 1984. pp. 325–348. [Google Scholar]
- Yildizhan S, Van Loon J, Sramkova A, Ayasse M, Arsene C, Schulz S. Aphrodisiac pheromones from the wings of the small cabbage white and large cabbage white butterflies, Pieris rapae and Pieris brassicae. ChemBioChem. 2009;10:1666–1677. doi: 10.1002/cbic.200900183. [DOI] [PubMed] [Google Scholar]
- Zuk M, Kolluru GR. Exploitation of sexual signals by predators and parasitoids. Quart. Rev. Biol. 1998;73:415–438. doi: 10.1086/420412. [DOI] [Google Scholar]