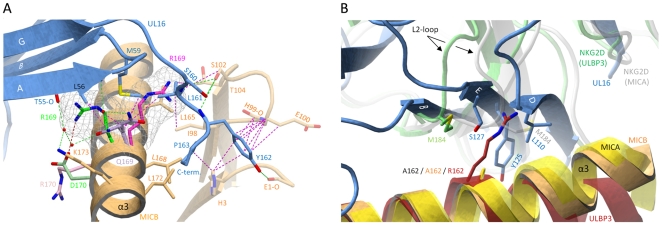
Figure 6. Selectivity of NKG2D ligand binding by UL16.
(A), The α1α2-platform domain of NKG2D-bound MICA [26] was superimposed onto MICBpf, but only the MICA side chains Arg170 (pink) and Arg169 (magenta) are shown. The α1α2-domain of NKG2D-bound ULBP3 [28] was also superposed onto MICB, and only the ULBP3 side chains of Arg169 (green) and Asp170 (light green) are shown. Cages surrounding the two arginines of MICA and ULBP3 at position 169 depict the area that these side chains would require in a space-filling model. In both cases, the arginine side chains would clash with UL16 residues. (B), The α1α2-platform domain of NKG2D-bound MICA [26] (yellow) and ULBP3 [28] (red), respectively, was superimposed onto MICBpf (orange). The side chains of alanine (present in MICBpf and MICA) and arginine (present in ULBP3) at position 162 are shown. Also shown are the Met184 side chains of both the MICA-bound (white) and ULBP3-bound (green) NKG2D monomers, both of which correspond to the green NKG2D monomer in Figures 5A and 5B. Conformational changes of the L2-loop of MICA-bound NKG2D displaces Met184 and allows for the accommodation of Arg162 in ULBP3-bound NKG2D. In UL16, the rigid DEB sheet does not allow for a similar conformational adjustment, and ULBP3 residue Arg162 would therefore clash with UL16 residues.