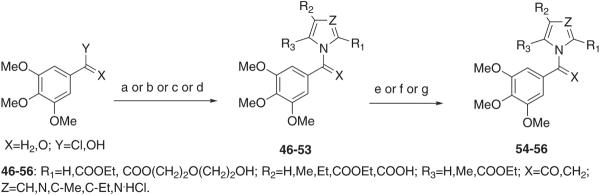
Scheme 6.
Synthesis of Compounds 46–56a
a Reagents and reaction conditions: (a) (46–49,X = H2,Y = Cl) appropriate ethyl 1H-pyrrole-2-carboxylate, 3,4,5-trimethoxybenzyl chloride, TBAHS, 50% KOH/DCM, 25 °C, overnight, 45–57%; (b) (50,X = H2,Y = OH) ethyl 1H-imidazole-4-carboxylate, 3,4,5-trimethoxybenzyl alcohol, P(Ph3)3, DIPAD, anhydrous THF, 25 °C, overnight, Ar stream, 10%; (c) (51, X = O,Y = Cl) ethyl 1H-pyrrole-2-carboxylate, 3,4,5-trimethoxybenzoyl chloride, 18-crown-6, t-BuOK, anhydrous THF, 25 °C, 4 h, 8%; (d) (52 and 53, X = O,Y = Cl) ethyl 1H-imidazole-4-carboxylate, 3,4,5-trimethoxybenzoyl chloride, anhydrous DCM, reflux temperature, 2.5 h, 13–15%; (e) (54) KOH, MeOH/H2O, reflux temperature, 3 h, 74%; (f) (55) diethylene glycol, KOH, 140 °C, 3 h, 34%; (g) (56) 37% HCl, reflux temperature, 1.5 h, 68%.