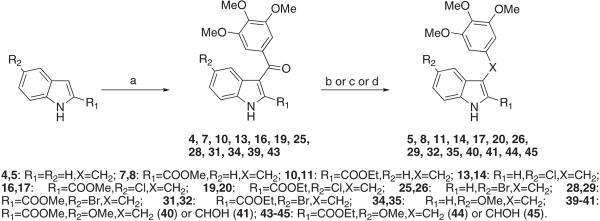
Scheme 1.
Synthesis of Compounds 4, 5, 7, 8, 10, 11, 13, 14, 16, 17, 19, 20, 25, 26, 28, 29, 31, 32, 34, 35, 39–41 and 43–45a
a Reagents and reaction conditions: (a) 3,4,5-trimethoxybenzoyl chloride, AlCl3, 1,2-dichloroethane, closed vessel, 110 °C, 150 W, Pmax = 250 PSI, 2 min, 40–68%; (b) (5, 14, 26, and 35) NaBH4 (10 equiv), EtOH, reflux, 3 h, 42–68%; (c) (8, 11, 17, 20, 29, 32, 41, and 45)Et3SiH, CF3COOH, 25 °C, overnight, 46–90%; (d) (40 and 44) NaBH4 (1 equiv), THF/H2O, reflux, 2 h, 29–53%.