Figure 3.
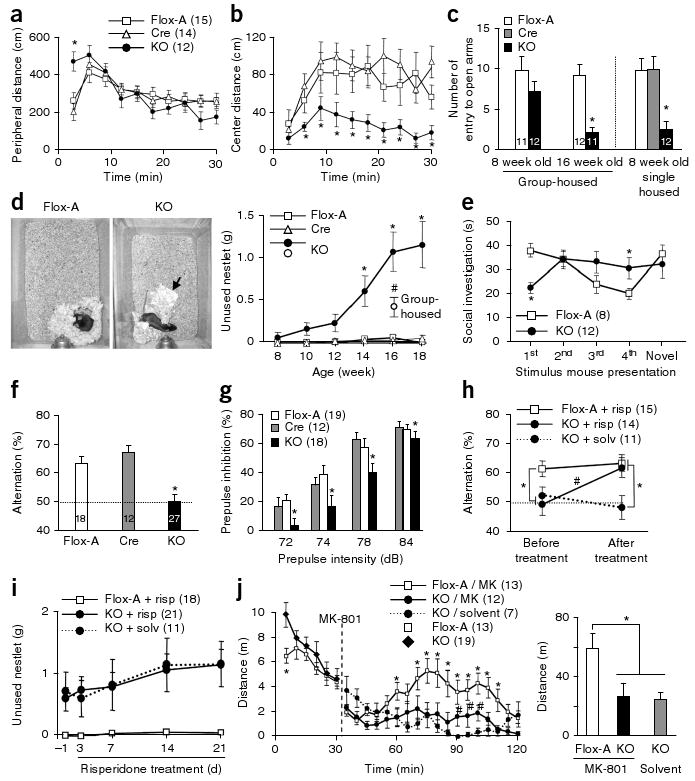
Early postnatal NR1 deletion leads to schizophrenia-related behaviors. (a,b) Increased peripheral locomotion following exposure to a novel open field and decreased exploration of the anxiogenic central area in mutant mice versus controls (Cre and Flox-A) (repeated measures ANOVA/LSD post hoc test, * P < 0.05 versus Flox-A and Cre). (c) Social isolation induced anxiety-like behavior in the elevated plus maze, as shown by the decreased number of entries into unprotected open arms by mutant mice compared with controls (ANOVA/LSD post hoc test, * P < 0.05 versus controls). (d) Left, representative pictures of the nests of Flox-A and mutant mice (arrow, unused material). Right, single-housed mutant mice displayed a significant deficit in nest construction starting at 14 weeks of age, as determined by weighing the unused nestlet material after overnight housing in a new cage (two-way ANOVA/LSD post hoc test, * P < 0.05 versus Flox-A and Cre). Group-housed mutant mice had a smaller deficit compared with age-matched single-housed mutants (16 weeks old, ANOVA/LSD post hoc test, # P < 0.05 versus controls and single-housed mutant mice). (e) The social-recognition test revealed impairment in social short-term memory in the mutant. Flox-A control mice decreased social investigation time following repeated exposures (1 min each) to a stimulus mouse. A fifth dishabituation trial elicited an increased response to a novel mouse, showing individual recognition. In contrast, mutant mice did not show habituation after repeated presentations of the stimulus mouse (second to fourth presentation, repeated measures ANOVA/LSD post hoc test, * P < 0.05 versus Flox-A), suggesting social amnesia, and reduced investigation time during the first presentation. (f) In the Y-maze spontaneous alternation task, the mutant mice alternated between the arms at the chance level (dotted line; ANOVA/LSD post hoc test, * P < 0.005 versus Flox-A and Cre). (g) Mutant mice at 10–12 weeks of age were impaired in prepulse inhibition of the auditory startle reflex across prepulse intensities (two-way ANOVA/LSD post hoc test, * P < 0.005 for genotype factor). (h) Chronic treatment with risperidone (2.5 mg per kg of body weight per d for 3 weeks in drinking water) ameliorated the working memory deficit of the mutant mice in the Y-maze spontaneous alternation task (repeated-measures ANOVA/LSD post hoc test, * P < 0.05, versus. Flox-A, # P < 0.001 mutant mice treated with risperidone versus solvent). Dotted line indicates chance level. (i) Chronic risperidone treatment did not rescue the deficit in social nest building (repeated-measures ANOVA/LSD post hoc test, P = 0.56 mutant mice treated with risperidone versus solvent, P < 0.01 mutant mice treated with risperidone versus Flox-A treated with risperidone). (j) Noncompetitive NMDAR antagonist–induced locomotion was diminished in mutant mice. Left, time course of MK-801–induced locomotor activity (broken line, MK-801 injection, 0.2 mg per kg intraperitoneal; repeated-measures ANOVA/LSD post hoc test, * P < 0.05 versus MK801-treated mutant mice (MK), # P < 0.05 versus mutant mice treated with solvent). Right, cumulative distance traveled after MK-801 treatment (30–120 min; one-way ANOVA/LSD post hoc test, * P < 0.02). Data are mean ± s.e.m.; n is indicated in parentheses or plot bars. Cre, Ppp1r2-cre+/−; Flox-A, NR1loxP/loxP-line A; KO, Ppp1r2-cre+/−; NR1loxP/loxP-line A.
