Figure 1.
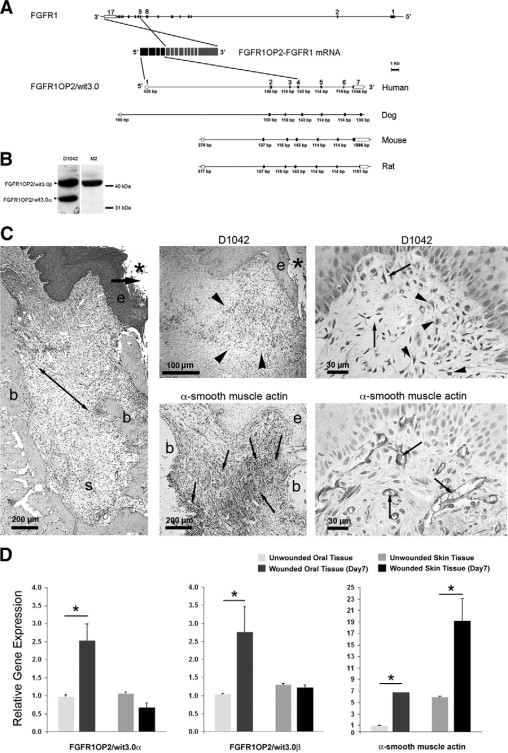
FGFR1OP2/wit3.0 and α-SMA in oral wound. A: Gene structures of FGFR1 and FGFR1OP2/wit3.0. In a case report of acute myeloid leukemia,23 the exons 1 to 4 of FGFR1OP2/wit3.0 were trans-inserted to the intron between exons 8 and 9 of FGFR1, and the resulting chimerical peptide formed a ligand-independent dimer through the FGFR1OP2/wit3.0-derived coiled-coil structure. The native FGFR1OP2/wit3.0 gene contains seven highly conserved exons. B: Western blot analysis of monospecific polyclonal antibody (D1042) recognized 41 kDa and 32 kDa bands, corresponding to FGFR1OP2/wit3.0β and FGFR1OP2/wit3.0α, respectively, in NIH fibroblasts. The transfected 3XFLAG-FGFR1OP2/wit3.0β fusion peptide was recognized by M2 monoclonal antibody against 3XFLAG epitope at 41 kDa. C: Oral open wound (asterisk) created by tooth extraction showed progressive wound contraction (arrows) by wound margin approximation (e: proliferation/migration front of oral epithelium; b: alveolar bone; s: tooth extraction socket, H&E staining). D1042 antibody stained a highly restricted zone of oral connective tissue (arrowheads) immediately adjacent to the migration/proliferation front of oral epithelium (e). α-SMA (small arrows) was identified along the ligament-like structure (double arrowhead in H&E stained section) connecting alveolar bones (b). D1042 stained the cytoplasma of fibroblasts with cuboidal shapes (arrowheads), whereas α-SMA was found in vascular smooth muscles (small arrows). D: Real-time RT-PCR revealed that FGFR1OP2/wit3.0α and FGFR1OP2/wit3.0β were up-regulated in oral wound tissues but not in skin wound tissues, whereas α-SMA was up-regulated in both oral and skin wound tissues albeit at different levels (*P < 0.05).
