Abstract
OBJECTIVE
Our aim was to study the recurrence risk of type 1 diabetes in the offspring of parents with adult-onset (15–39 years) type 1 diabetes and to evaluate the transmission of diabetes within a continuum of parental age at onset of diabetes from childhood to adulthood.
RESEARCH DESIGN AND METHODS
Diabetes status of all offspring (n = 9,636) in two Finnish cohorts of parents with type 1 diabetes was defined until the end of year 2007. Cumulative incidences of type 1 diabetes among the offspring were estimated, and several factors contributing to the risk were assessed.
RESULTS
During 137,455 person-years, a total of 413 offspring were diagnosed with type 1 diabetes. The cumulative incidence by 20 years was 4.0% (95% CI 3.1–4.8) for the offspring of parents with adult-onset diabetes. The risk was equal according to the sex of the parents. The cumulative incidence decreased in parallel with the increase in age at onset of diabetes in the fathers. In the offspring of diabetic mothers, the risk was equal regardless of the age at onset of diabetes. However, the reduced risk in the maternal offspring was most pronounced in the daughters of the mothers with a diagnosis age <10 years.
CONCLUSIONS
Type 1 diabetes transmission ratio distortion is strongly related to the sex and age at onset of diabetes in the diabetic parents.
Type 1 diabetes can occur at any age, although it is predominantly seen in children and young adults. Therefore, the majority of studies have been conducted in children aged <15 years. The recurrence risk in the offspring ranges from 3 to 6% depending on the study design, follow-up time, and the population where the study was conducted (1–3). Little is known about the recurrence risk in first-degree relatives of subjects diagnosed with type 1 diabetes, aged >15 years. The incidence of type 1 diabetes is much lower in young adults than in children (4–6). Consequently, the risk of family members may also be different among the diabetic subjects affected after childhood.
Sex-related factors seem to be involved in the transmission of diabetes from one generation to the next (7). By 20 years of age, 5–8% of the offspring of diabetic men and only 2–5% of the offspring of diabetic women have been found to be affected (1–3,8). We have previously shown that the recurrence risk of diabetes in the offspring of parents diagnosed between 0–17 years of age was higher the younger the father was when diagnosed with type 1 diabetes. This pattern was not present in the offspring of the mothers (8). However, it is not known whether the sex-related factors play a role in the transmission of diabetes in adult-onset type 1 diabetes. We have now enlarged our study to also include the offspring of parents diagnosed with diabetes between 15 and 39 years of age. This gives us an opportunity to determine the risk in the offspring of parents with a broad age span at diagnosis and to elucidate whether there are differences in the risk between the offspring of diabetic mothers and fathers.
RESEARCH DESIGN AND METHODS
All offspring of Finnish population-based cohorts of probands with late-onset type 1 diabetes diagnosed between 15 and 39 years of age were identified and followed regarding a diagnosis of type 1 diabetes until the end of year 2007. This cohort comprised 3,389 subjects diagnosed during 1992–2001. This cohort was originally used for the study of incidence rates of type 1 and type 2 diabetes in Finland, and the ascertainment procedure has been previously described (4,5). Moreover, we used the same cohort of patients with early-onset diabetes diagnosed before the age of 18 years between 1965 and 1979 (n = 5,144) as in our previous publication in order to re-examine whether the recurrence risk of type 1 diabetes in the offspring of mothers occurs in a sex-specific manner—the observation that was only suggestive in our previous publication (8). The analysis was conducted by means of increasing statistical power and taking into consideration parental age at onset. We identified all new offspring born between 2002 and 2006 (n = 520) and updated the diabetes status of the offspring until the year 2007.
The early-onset cohort was also used to provide a holistic view on the effect of parental onset of diabetes on the recurrence risk of type 1 diabetes in the offspring. All offspring were identified through the Central Population Register and linked to the National Hospital Discharge Register, the Drug Reimbursement Register, and the nationwide Finnish Diabetes Register of the National Institute of Health and Welfare.
Statistical methods.
The analyses were first carried out in the late-onset cohort. Cox regression analysis was used to assess factors associated with the risk of progression to type 1 diabetes, and the following potential predictors were included in the analyses: sex of the parent, sex of the offspring, age at the onset of parental diabetes as a continuous variable, year of birth of the offspring, and parental age at delivery. At the first stage, univariate analyses were performed. At the second stage, standard backward selection and stepwise selection techniques were used to identify possible significant variables. All variables were included in the full model irrespective of statistical significance in the univariate analysis. A likelihood ratio test was used as a test statistic in the comparison of the models at each step. Interactions between the variables were also tested.
The risk of diabetes in the offspring of the mothers diagnosed before (n = 875) and after (n = 642) the offspring's birth was also tested. In this analysis, the offspring of the mothers diagnosed between 20 and 39 years of age were included.
The effect of the parental age at diagnosis on the risk of diabetes in the offspring was evaluated by pooling the data on the early- and late-onset cohorts. Kaplan-Meier analyses provided long-term cumulative risk for the development of type 1 diabetes in the offspring. In order to find out whether the risk, associated with the age at onset of the fathers, levels off at some stage, the offspring were stratified into 5-year groups by paternal age at onset, and hazard ratios (HRs) were calculated using the last group as reference.
Finally, because there were conspicuously fewer affected girls in the two lowest age-at-onset groups among the mothers, the data were divided into two groups: the mothers' age at diagnosis <10 years and ≥10 years, and the risk difference in the sons and daughters was tested.
RESULTS
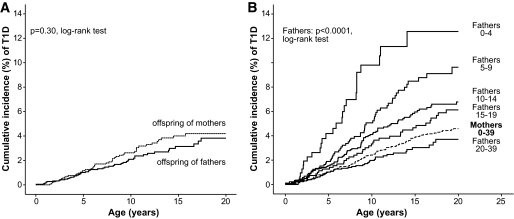
Altogether there were 9,696 offspring in the two cohorts: 3,881 in the late-onset cohort and 5,821 in the early-onset cohort (13 overlapped) (Table 1). The offspring in the late-onset cohort were ∼5.2 years younger than those in the early-onset cohort. During 137,455 person-years of observation, a total of 413 offspring were diagnosed with type 1 diabetes: 318 offspring of parents from the early-onset cohort and 97 offspring of parents from the late-onset cohort (two overlapped). The overall incidence rate was 241.6 per 100,000 person years (95% CI 201.5–287.2) in the offspring of parents diagnosed between 15 and 39 years of age. The cumulative risk by 20 years was 4.0% (3.1–4.8) for the offspring of parents with late-onset diabetes. The risk in the offspring did not differ by sex of the parents (P = 0.30); the 20-year cumulative incidence was 4.2% (3.0–5.4) for the offspring of mothers and 3.8% (2.6–5.0) for the offspring of fathers (Fig. 1A).
TABLE 1.
Descriptive data on the study population
| Female probands 0–17 | Male probands 0–17 | All probands 0–17 | Female proband 15–39 | Male proband 15–39 | All proband 15–39 | All proband 0–39 | |
|---|---|---|---|---|---|---|---|
| Offspring | 2,501* | 3,333* | 5,821* | 1,667 | 2,214 | 3,881 | 9,696† |
| Girls | 1,248 | 1,615 | 2,857 | 809 | 1,108 | 1,961 | 4,771 |
| Boys | 1,253 | 1,718 | 2,964 | 858 | 1,106 | 1,915 | 4,925 |
| Offspring with T1D | 100 | 218 | 318 | 50 | 47 | 97 | 413‡ |
| Girls | 45 | 99 | 144 | 21 | 29 | 50 | 194 |
| Boys | 55 | 119 | 174 | 29 | 18 | 45 | 219 |
Fourteen offspring with type 1 diabetes were excluded from the analyses because they had both parents with type 1 diabetes.
*Thirteen offspring had both parents from the early-onset cohort;
†six overlapped offspring with 2 diabetics; and
‡two overlapped offspring with type 1 diabetes. T1D, type 1 diabetes.
FIG. 1.
Cumulative incidence of type 1 diabetes (T1D) in the offspring of fathers and mothers with late-onset type 1 diabetes (P = 0.30, log-rank test) (A) and in the offspring of fathers in the combined cohorts according to age at onset of diabetes in the fathers (P < 0.0001, log-rank test) and in the offspring of mothers in the combined cohorts (P = 0.69, log-rank test) (B).
Cox proportional hazard modeling revealed that none of the tested variables was associated within the recurrence risk in the offspring of the parents with late-onset diabetes, neither in univariate nor in any step in multivariate analyses (Table 2).
TABLE 2.
Multivariate Cox regression analysis including factors that influence the recurrence risk in offspring of parents with late-onset type 1 diabetes
| Univariate analyses |
Multivariate analyses (full model) |
|||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P | Adjusted HR | 95% CI | P |
| Sex of diabetic parent | ||||||
| Female | 1.00 | — | ||||
| Male | 0.81 | 0.54–1.21 | 0.30 | 0.78 | 0.52–1.18 | 0.23 |
| Sex of offspring | ||||||
| Female | 1.00 | — | 1.00 | — | ||
| Male | 0.88 | 0.59–1.31 | 0.52 | 0.87 | 0.58–1.30 | 0.49 |
| Age at onset of diabetes in parents | ||||||
| Continuous | 0.97 | 0.93–1.00 | 0.06 | 0.97 | 0.91–1.04 | 0.42 |
| Year of birth of offspring | ||||||
| Continuous | 1.04 | 1.00–1.08 | 0.06 | 1.02 | 0.95–1.10 | 0.57 |
| Parental age at delivery | 1.01 | 0.96–1.05 | 0.81 | 1.01 | 0.94–1.09 | 0.78 |
No significant interaction terms.
There was no difference between the recurrence risk in the offspring of the mothers when pregnancy occurred before versus after the diagnosis of the maternal diabetes (P = 0.22). HR was 1.5 (95% CI 0.8–3.0) for the offspring born to the mothers with diabetes compared with those born before the mother's diabetes.
HR for the offspring of the fathers diagnosed at 15–19 years of age was 2.9 (95% CI 1.3–6.7) compared with those diagnosed at 35–39 years of age. The risk in the diagnosis groups (20–24, 25–29, and 30–34 years) was similar as in the reference group. Thus, in the Kaplan-Meier analyses, the offspring with paternal diagnosis aged 20–39 years were pooled.
When the offspring of the two cohorts were pooled and analyzed according to age at onset of diabetes in the parents, the risk of the offspring of the fathers showed a decreasing risk trend by the increase in the father's age at onset (Fig. 1B). In contrast to the offspring of fathers, the risk was equal in the offspring of the mothers in all age-at-onset groups. In the offspring of the mothers, the 20-year cumulative risk was 4.7% (95% CI 3.9–5.4) irrespective of the age at onset of the mothers (Fig. 1B).
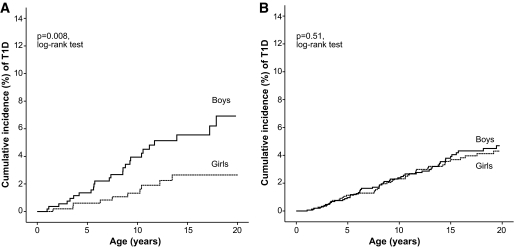
However, a closer examination revealed that the risk in the sons of the mothers affected before age <10 years was 2.0 times higher (95% CI 1.0–3.6, P = 0.008) compared with the risk in the daughters (Fig. 2). Until 20 years, 2.7% (1.0–4.2) of the daughters were affected, while 6.9% (4.2–9.6) of the sons were affected.
FIG. 2.
Cumulative incidence of type 1 diabetes (T1D) in the boys and girls of the diabetic mothers with diabetes onset <10 years (P = 0.008, log-rank test) (A) and ≥10 years (P = 0.75, log-rank test) (B).
There was no difference between the sex of the offspring when the mothers' age at diagnosis was >10 years (P = 0.51) or overall in the offspring of the fathers (P = 0.92).
DISCUSSION
This study shows that in contrast to the data from early-onset type 1 diabetes, the recurrence risk is similar in the offspring of mothers and fathers with late-onset type 1 diabetes. The cumulative incidence decreased in parallel with the increase in age at onset of diabetes in the fathers. However, there was no such pattern in the offspring of the mothers, although age at onset <10 years in the mothers also had an enhancing impact on the recurrence risk in the sons. On the contrary, the daughters of the mothers with diabetes and young age at onset seem to be protected from diabetes.
This is the first longitudinal population-based study of the recurrence risk in offspring of parents with type 1 diabetes that takes into account the broad continuum of age at onset of diabetes in the parents from early childhood into adulthood. In general, most studies have focused on diabetes in children, and there are very few studies including adult-onset type 1 diabetes. However, it is obvious that a broad age span is essential in order to obtain a comprehensive picture of the risk pattern.
In many chronic diseases, age at onset is an indicator of genetic susceptibility. The stronger the genetic component, the earlier the onset of the disease and the greater the risk in the first-degree relatives (9,10). The present study supports such an age-related relationship. There are great genetic, autoimmune, and clinical differences between childhood-onset and adult-onset type 1 diabetes (11). These differences seem to be reflected in the heterogeneity of the transmission pattern of type 1 diabetes according to age at onset. It can be hypothesized that the risk pattern observed in the offspring of the diabetic fathers is the anticipated one, but that the reduced risk of the offspring of the diabetic mothers is unexpected.
There are at least two explanations for the reduced risk in the offspring of diabetic mothers. The offspring either may be protected from diabetes or may be selectively lost during pregnancy (12). Accumulating evidence indicates that the intrauterine environment affects health later in life (13). The phenomenon is referred to as fetal programming or epigenetic modification (14,15). Several studies have shown that a number of gestational events may contribute to the risk of type 1 diabetes (16–19). Boys seem to be more vulnerable to certain gestational events suggesting that perinatal determinants may influence the risk of diabetes in a sex-specific manner (20). It is noteworthy that an abnormal intrauterine environment may also protect the fetus through modification of gene expression of susceptibility genes for type 1 diabetes and that a fetus, exposed to maternal type 1 diabetes, could as a result be protected from the development of diabetes. Autoantibodies transmitted from the mother to the child have been reported to confer protection from the development of autoantibodies and type 1 diabetes later in life among children born to mothers with type 1 diabetes (21). It is, however, unclear whether these autoantibodies per se protect the offspring from diabetes or whether they are mere markers of other protective factors or mechanisms.
It has been suggested that one way to test the hypothesis that pregnancy may protect from diabetes is to compare the manifestation of diabetes in the offspring between those born before and after the mother has been diagnosed with diabetes (12,22). We did not find any differences between the two groups, and our results are thus consistent with those by Lorenzen et al. (2). However, it has to be acknowledged that a possible protection, if there is one, may only occur in the mothers with childhood and adolescent diabetes and possibly with certain combinations of susceptibility genes and antibodies, since the risk in the offspring of fathers and mothers affected with diabetes in adulthood is no different. Thus, it is not possible to conclude anything about the possible protective effect with this kind of comparison.
Although the capture years (1965–1979 and 1992–2001) at first glance seem very different, the age distribution of the parents in the two included cohorts was in fact similar, as were the birth year distributions of the offspring. In the early-onset cohort, offspring were born between 1969 and 2006; in the late-onset cohort, they were born between 1973 and 2006. The mean birth year in the first was 1990, and in the latter, it was 1995. Therefore, the lower risk of diabetes in the offspring in the late-onset cohort is probably due to other factors rather than the birth year.
A limitation is the lack of precise information about the type of diabetes in the offspring. Although type 2 diabetes has become increasingly common in children, it has to be emphasized that type 2 diabetes in subjects aged <20 years has been very rare in Finland during the past decades (4,5). The majority of the affected children were diagnosed before <20 years of age, and therefore type 1 diabetes was the most likely diagnosis.
In conclusion, the transmission ratio distortion is strongly related to the age at onset of diabetes in the diabetic parent. However, the recurrence risk is equal in the offspring of mothers and fathers with adult-onset diabetes.
Acknowledgments
This study was supported by grants from the Juvenile Diabetes Research Foundation, the Folkhälsan Research Foundation, and the Wilhelm and Else Stockmann Foundation.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Warram JH, Krolewski AS, Gottlieb MS, Kahn CR: Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med 1984;311:149–152 [DOI] [PubMed] [Google Scholar]
- 2.Lorenzen T, Pociot F, Stilgren L, Kristiansen OP, Johannesen J, Olsen PB, Walmar A, Larsen A, Albrechtsen NC, Eskildsen PC, Andersen OO, Nerup J; Danish IDDM Epidemiology and Genetics Group Predictors of IDDM recurrence risk in offspring of Danish IDDM patients. Diabetologia 1998;41:666–673 [DOI] [PubMed] [Google Scholar]
- 3.el-Hashimy M, Angelico MC, Martin BC, Krolewski AS, Warram JH: Factors modifying the risk of IDDM in offspring of an IDDM parent. Diabetes 1995;44:295–299 [DOI] [PubMed] [Google Scholar]
- 4.Lammi N, Taskinen O, Moltchanova E, Notkola IL, Eriksson JG, Tuomilehto J, Karvonen M: A high incidence of type 1 diabetes and an alarming increase in the incidence of type 2 diabetes among young adults in Finland between 1992 and 1996. Diabetologia 2007;50:1393–1400 [DOI] [PubMed] [Google Scholar]
- 5.Lammi N, Blomstedt PA, Moltchanova E, Eriksson JG, Tuomilehto J, Karvonen M: Marked temporal increase in the incidence of type 1 and type 2 diabetes among young adults in Finland. Diabetologia 2008;51:897–899 [DOI] [PubMed] [Google Scholar]
- 6.Harjutsalo V, Sjöberg L, Tuomilehto J: Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 2008;371:1777–1782 [DOI] [PubMed] [Google Scholar]
- 7.Gale EA, Gillespie KM: Diabetes and gender. Diabetologia 2001;44:3–15 [DOI] [PubMed] [Google Scholar]
- 8.Harjutsalo V, Reunanen A, Tuomilehto J: Differential transmission of type 1 diabetes from diabetic fathers and mothers to their offspring. Diabetes 2006;55:1517–1524 [DOI] [PubMed] [Google Scholar]
- 9.Gillespie KM, Gale EA, Bingley PJ: High familial risk and genetic susceptibility in early onset childhood diabetes. Diabetes 2002;51:210–214 [DOI] [PubMed] [Google Scholar]
- 10.Harjutsalo V, Podar T, Tuomilehto J: Cumulative incidence of type 1 diabetes in 10,168 siblings of Finnish young-onset type 1 diabetic patients. Diabetes 2005;54:563–569 [DOI] [PubMed] [Google Scholar]
- 11.Hirschhorn JN: Genetic epidemiology of type 1 diabetes. Pediatr Diabetes 2003;4:87–100 [DOI] [PubMed] [Google Scholar]
- 12.Warram JH, Krolewski AS, Kahn CR: Determinants of IDDM and perinatal mortality in children of diabetic mothers. Diabetes 1988;37:1328–1334 [DOI] [PubMed] [Google Scholar]
- 13.Eriksson JG: The role of genes in growth and later health. Nestle Nutr Workshop Ser Pediatr Program 2008;61:69–77 [DOI] [PubMed] [Google Scholar]
- 14.Vaissière T, Sawan C, Herceg Z: Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat Res 2008;659:40–48 [DOI] [PubMed] [Google Scholar]
- 15.Miao F, Smith DD, Zhang L, Min A, Feng W, Natarajan R: Lymphocytes from patients with type 1 diabetes display a distinct profile of chromatin histone H3 lysine 9 dimethylation: an epigenetic study in diabetes. Diabetes 2008;57:3189–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leslie RD, Elliott RB: Early environmental events as a cause of IDDM: evidence and implications. Diabetes 1994;43:843–850 [DOI] [PubMed] [Google Scholar]
- 17.Stene LC, Magnus P, Lie RT, Sovik O, Joner G: Maternal and paternal age at delivery, birth order, and risk of childhood onset type 1 diabetes: population based cohort study. BMJ 2001;323:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahlquist GG, Patterson C, Soltesz G: Perinatal risk factors for childhood type 1 diabetes in Europe: the EURODIAB Substudy 2 Study Group. Diabetes Care 1999;22:1698–1702 [DOI] [PubMed] [Google Scholar]
- 19.Dahlquist GG, Pundzīute-Lyckå A, Nyström LSwedish Childhood DiabetesStudy Group, Diabetes Incidence Study in Sweden (DISS) Group Birthweight and risk of type 1 diabetes in children and young adults: a population-based register study. Diabetologia 2005;48:1114–1117 [DOI] [PubMed] [Google Scholar]
- 20.Elfving M, Svensson J, Oikarinen S, Jonsson B, Olofsson P, Sundkvist G, Lindberg B, Lernmark A, Hyöty H, Ivarsson SA: Maternal enterovirus infection during pregnancy as a risk factor in offspring diagnosed with type 1 diabetes between 15 and 30 years of age. Exp Diabetes Res 2008;2008:271958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koczwara K, Bonifacio E, Ziegler AG: Transmission of maternal islet antibodies and risk of autoimmune diabetes in offspring of mothers with type 1 diabetes. Diabetes 2004;53:1–4 [DOI] [PubMed] [Google Scholar]
- 22.Tillil H, Köbberling J: Age-corrected empirical genetic risk estimates for first-degree relatives of IDDM patients. Diabetes 1987;36:93–99 [DOI] [PubMed] [Google Scholar]