Fig 1. Mutated VE-EC1-4 fragments.
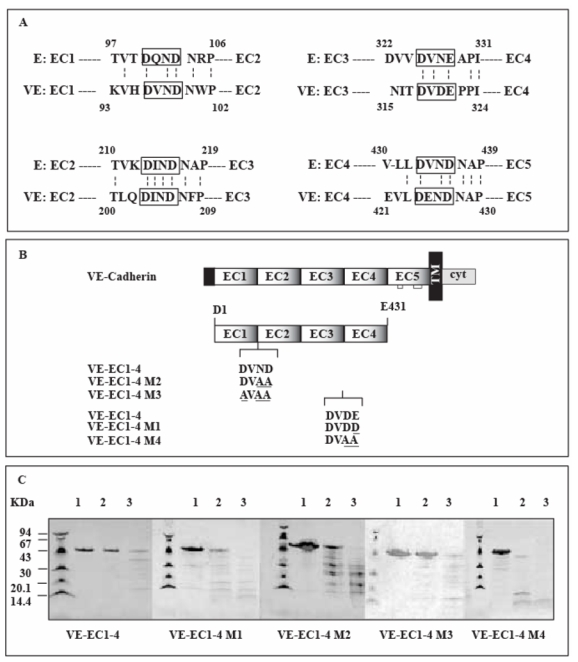
The extracellular region of cadherins consists of five homologous domains designated EC1 to EC5.
Panel A: Alignment of the extracellular parts of murine E- and human VE-cadherins
The four amino acid sequences linking the EC domains for mouse E. and human VE- cadherins are aligned. The conserved structural motifs between adjacent EC domains are boxed. Dotted vertical bars join amino acids conserved in both cadherin sequences. The numbers refer to the amino acids of E- and VE-cadherin according to the published sequences (GenBank P09803 and P33151 for mouse E- and human VE-cadherin, respectively)
Panel B : VE-cadherin mutants expressed in E. coli
VE-cadherin and its derived fragment VE-EC1-4 are schematically represented at the top. Mutations affecting conserved structural motifs, localized at the VE-EC1-4 inter-module sequences, are underlined. For comparison, the wild-type motifs from VE-cadherin are also mentionned. The name of each mutant is given at the left margin.
Panel C : Trypsin digestion of VE-cadherin mutants
Mutated VE-EC1-4 fragments were digested during 20 min at 22°C by trypsin using enzyme/fragment ratios of 0 (lane 1), 1.7 (lane 2), 13.6 (lane 3) U/μM. Molecular weight markers are at the left side of each gel. Trypsin-digested fragments were run through 8-25 % gels (Phast System, Amersham-Pharmacia). Following migration, proteins were detected by Coomassie blue staining.
