Fig 6. Cell-cell contacts initiated by expressed VE-Cad-GFP variants in CHO cells.
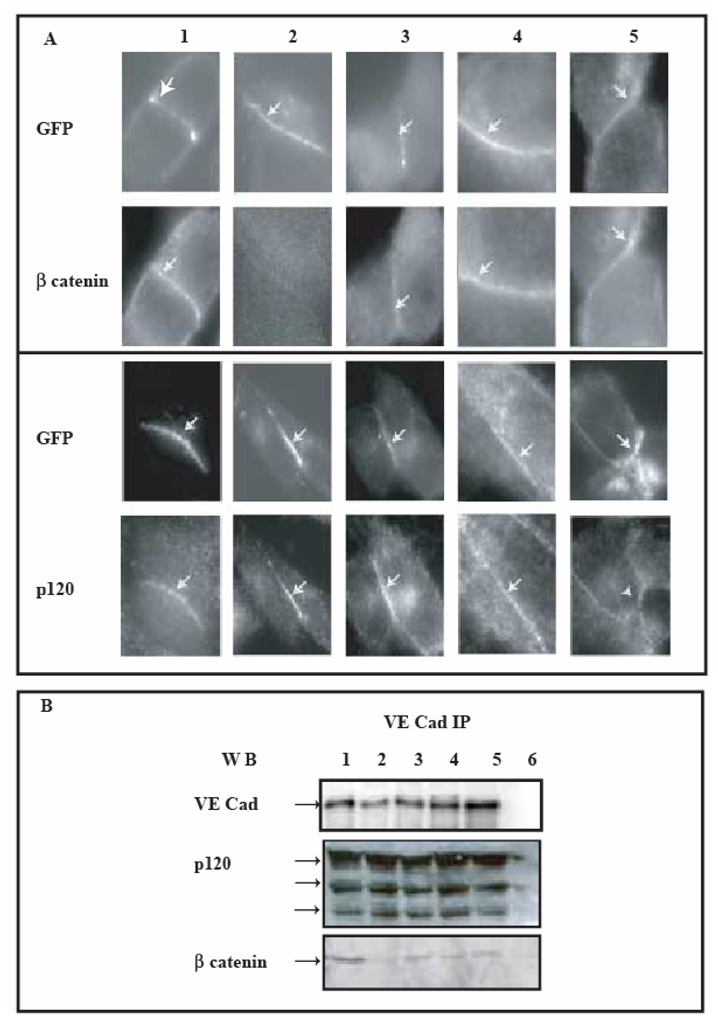
The ability of transfected cells to form a cadherin-based adhesion was assessed by immunofluorescence microscopy and immunoprecipitation methods. These experiments were performed 3h after serum-starvation arrest.
Panel A: Colocalization of VE-cadherin-GFP variants with G-catenin and p120 at intercellular junctions
VE-cadherin-derived proteins were observed using the intrinsic fluorescence of GFP whereas β-catenin and p120 were both immuno-fluorescently detected at the surface of transiently transfected CHO cells expressing VE-Cad-GFP: lane 1, VE-Cadtr-GFP: lane 2, VE-CadM1-GFP: lane 3, VE-CadM2-GFP: lane 4, VE-CadM3-GFP: lane 5. VE-cadherin-GFP variants and β-catenin or p120 colocalized, except for VE-Cadtr-GFP which failed to recruit β-catenin.
Panel B: Cytoplasmic partners of VE-cadherin-GFP variants
Lysates of CHO cells expressing VE-Cad-GFP (lane 1), VE-Cadtr-GFP (lane 2), VE-CadM1-GFP (lane 3), VE-CadM2-GFP (lane 4), VE-CadM3-GFP (lane 5) were immunoprecipitated with an Anti-VE-cadherin antibody. VE-cadherin immunoprecipitates were alternatively blotted with antibodies to either VE-cadherin, p120 and β-catenin. As a control, immunoprecipitation of untransfected CHO cell lysates was blotted in parallel (lane 6).
