Figure 4.
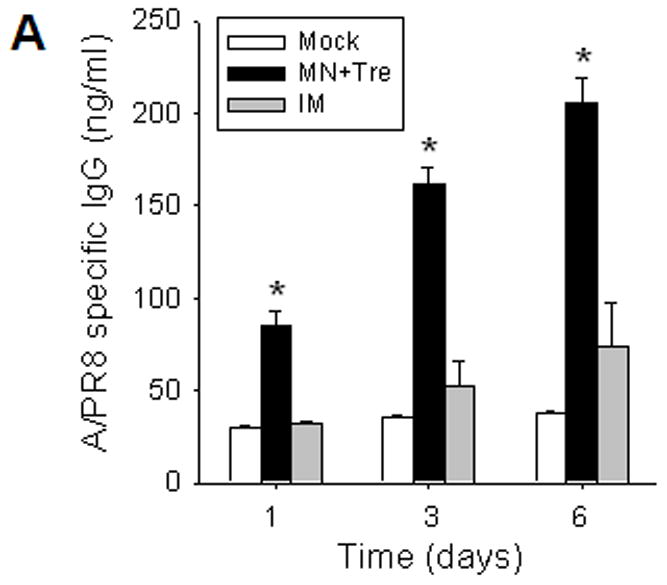
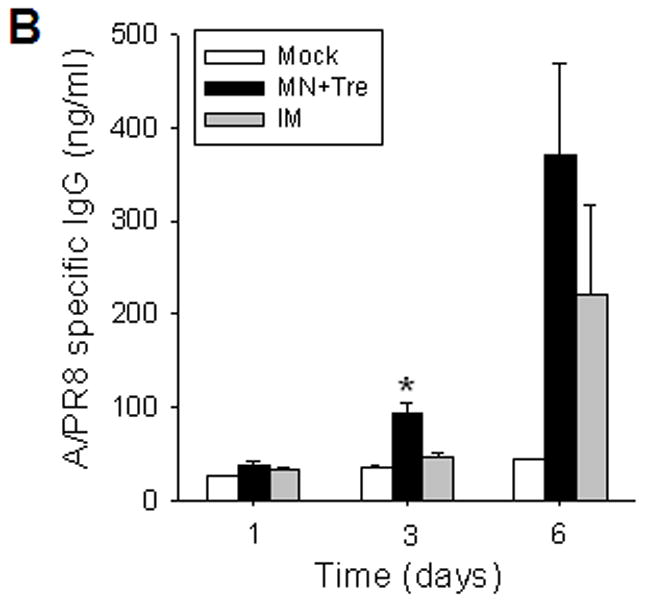
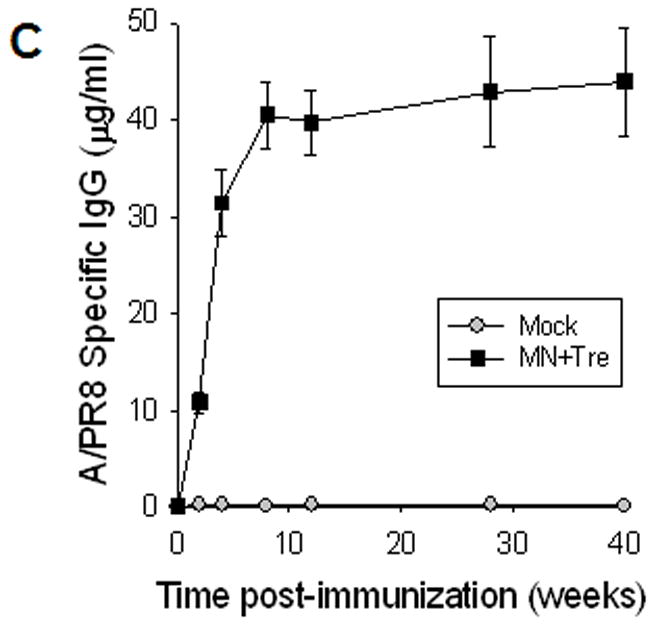
Rapid recall and long-term immune responses. Bone marrow and spleen cells were harvested at day 4 post challenge (n=4), and kinetics of virus-specific IgG antibody production were determined. Antibody levels in the fourfold diluted in vitro culture supernatants were determined by ELISA (OD at 450 nm) after 1 to 6 days of incubation, and were expressed in concentrations (ng/ml) using standard mouse antibodies. (A) Bone marrow cell cultures (5 × 105 cells/well) in the absence of influenza virus antigen stimulation (n=4, *p<0.05). (B) Spleen cell cultures (5 × 105 cells/well) in the plate coated with inactivated influenza viral antigen (n=4, *p<0.005). Groups of mice (n=4, *p<0.05) are as described in the legend of Figure 2. (C) Long-term maintenance of antibody levels by microneedle vaccination. In an independent experiment for long-term antibody responses, virus specific antibody responses were determined over a 9 month period in mice (n=6) immunized in the skin with trehalose-formulated microneedle vaccine (0.7 μg inactivated influenza virus). Time 0 is the IgG value from the serum samples obtained before immunization of mice with microneedle vaccine. Serial diluted serum samples were used for ELISA and antibody levels were expressed in concentrations (μg/ml) from a mouse antibody standard curve. *: MN+Tre compared with Mock and IM.
