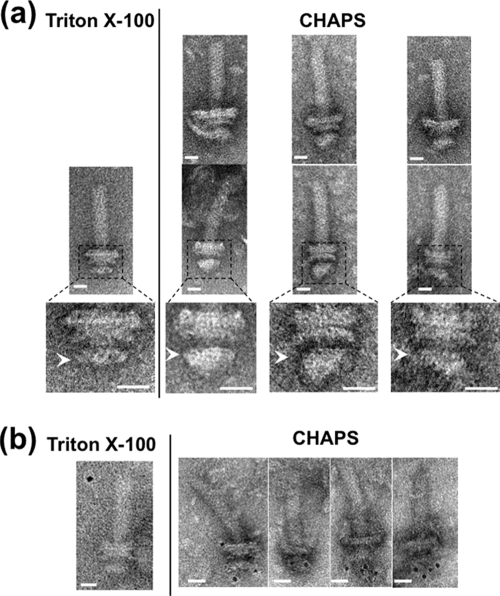
FIG. 3.
Electron micrographs of basal bodies isolated using CHAPS. (a) Comparison of electron micrographs of basal bodies isolated by CHAPS (right) and by Triton X-100 (left). Six representative images are shown for CHAPS-solubilized basal bodies in fraction 13 (Fig. 2) of sucrose density gradient (upper and middle). Enlarged images of the middle panel corresponding to the dotted frame are shown in the lower panels. Arrowheads indicate the MS rings. The samples containing flagellar structures were put on a hydrophilic-treated carbon-coated copper grid. The grids were washed with TE buffer, negatively stained with 3% uranyl acetate and observed with a JEM-1200EX or JEM-2010 electron microscope (JEOL, Japan). Bar, 20 nm. (b) Immunogold labeling of the basal bodies isolated by CHAPS (right) and by Triton X-100 (left) using anti-FliG antibody. 3 μl of the basal body suspension was mixed with 1 μl of buffer A (10 mM Tris, 5 mM EDTA, 600 mM NaCl at pH 8.0) and with 1 μl of the antibody solution diluted 20-fold with buffer B (10 mM Tris, 5 mM EDTA, 150 mM NaCl at pH 8.0). The mixture was kept on ice for 10 min. One μl of 5-nm colloidal gold-conjugated anti-rabbit IgG antibody diluted 2-fold with buffer C (10 mM Tris, 5 mM EDTA, 300 mM NaCl at pH 8.0) was then added to this mixture and it was left at room temperature for 30 min. Next, the mixture was put on a hydrophilic-treated carbon-coated copper grid. The grids were washed twice with buffer (10 mM Tris-HCl [pH 8.0], 5 mM EDTA), negatively stained with 3% uranyl acetate, and observed with a JEM-1200EX or JEM-2010 electron microscope (JEOL, Japan). Bar, 20 nm.