Abstract
Hepatitis B and C viruses (HBV and HCV, respectively) are different and distinct viruses, but there are striking similarities in their disease potential. Infection by either virus can cause chronic hepatitis, liver cirrhosis, and ultimately, liver cancer, despite the fact that no pathogenetic mechanisms are known which are shared by the two viruses. Our recent studies have suggested that replication of either of these viruses upregulates a cellular protein called serine protease inhibitor Kazal (SPIK). Furthermore, the data have shown that cells containing HBV and HCV are more resistant to serine protease-dependent apoptotic death. Since our previous studies have shown that SPIK is an inhibitor of serine protease-dependent apoptosis, it is hypothesized that the upregulation of SPIK caused by HBV and HCV replication leads to cell resistance to apoptosis. The evasion of apoptotic death by infected cells results in persistent viral replication and constant liver inflammation, which leads to gradual accumulation of genetic changes and eventual development of cancer. These findings suggest a possibility by which HBV and HCV, two very different viruses, can share a common mechanism in provoking liver disease and cancer.
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are serious worldwide health problems, with more than 500 million people believed to be chronically infected with at least one of these viruses (36). HBV is a DNA virus belonging to the Hepadnaviridae family (21), while HCV is an RNA virus belonging to the Flaviviridae family (7). Despite the fact that they are two very different viruses, they share a common pathology in the ability to cause chronic hepatitis, liver cirrhosis, and ultimately, hepatocellular carcinoma (HCC) (34). It remains unclear why these two viruses, which are fundamentally so different, can both lead to similar disease states and the development of HCC.
Numerous studies suggest that in chronic viral hepatitis, the host's immune system is unable to clear infected cells (34). The persistent viral replication further stimulates liver inflammation, and prolonged inflammation and viral persistence result in a gradual accumulation of genetic changes which can subsequently lead to transformation and development of HCC (3, 13). It is possible that part of this failure of the host to clear infected cells results from an inability to induce apoptosis in these cells. For example, persistent HBV/HCV infection suppresses cytotoxic-T-lymphocyte (CTL)-induced apoptosis (3, 4). Apoptosis, or programmed cell death, plays a critical role in embryonic development, immune system function, and the overall maintenance of tissue homeostasis in multicellular organisms. It is also important in the host's control of viral infection (4). The execution of the apoptotic program has traditionally been considered the result of the activation of a family of proteases known as caspases. Caspase-dependent cell apoptosis (CDCA) usually initiates by activating caspases 8 and 10 through proteolysis of their proenzymes, which further activates the executioner caspases, such as caspase 3 and caspase 7, resulting in the degradation of chromosomal DNA and cell death (28, 29). Recent evidence, however, has suggested that apoptotic cell death can also be promoted and triggered by serine proteases in a caspase-independent manner (5, 6, 39). Serine protease-dependent cell apoptosis (SPDCA) differs from CDCA in that serine proteases, not caspases, are critical to the apoptotic process (1, 6, 39). Interestingly, certain viral infections have been shown to induce SPDCA (27, 39).
Failure of the immune-mediated removal of malignant cells through apoptosis may be due to the upregulation of apoptosis inhibitors in these cells (12, 18). We recently demonstrated that SPDCA can be inhibited by a small, 79-amino-acid protein called serine protease inhibitor Kazal (SPIK) (22). SPIK, which is also known as SPINK1, TATI (tumor-associated trypsin inhibitor), and PSTI (pancreas secretory trypsin inhibitor) (8, 24, 38), was first discovered in the pancreas as an inhibitor of autoactivation of trypsinogen (9). The expression of SPIK in normal tissue is limited or inactivated outside the pancreas, but expression of SPIK is elevated in numerous cancers, such as colorectal tumors, renal cell carcinoma, gastric carcinoma, and intrahepatic cholangiocarcinoma (ICC) (16, 19, 24, 31, 40, 41). It remains unknown, however, what role SPIK may play in cancer formation and development. Additionally, overexpression of SPIK was also found in HBV/HCV-infected human livers (32), and an even higher level of expression of SPIK was found in HBV/HCV-associated HCC tissue (19, 31). This implies that SPIK may be closely associated with hepatitis virus infection and development of HCC.
Here we show direct evidence that HBV/HCV replication does in fact upregulate expression of the apoptosis inhibitor SPIK, resulting in resistance to SPDCA, which could ultimately lead to the development of chronic hepatitis and liver cancer.
MATERIALS AND METHODS
Cell lines, plasmids, and small interfering RNA (siRNA) silencer.
The HCV replicon cell line G54, which consistently expresses the HCV 1a genome (11), and its parental cell line Huh7T, which is a cell line derived from Huh7 cells, were kindly provided by Baohua Gu and Tianlun Zhou (Institute for Hepatitis and Virus Research, Doylestown, PA [currently with Novartis, Shanghai, China]). The HCV replicon cell line 913 and its parental cell line were provided by Jutao Guo (Drexel University, PA). Cells were cultured and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum.
siRNA plasmids L71 and L183, expressing SPIK siRNA, were constructed using the pSilencer1.0 vector (Ambion, Austin, TX). SPIK siRNA expression was controlled by a U6 RNA polymerase III promoter. To generate SPIK siRNA, SPIK-specific 19-base oligonucleotides (71CAGGCATCTTTCTTCTCAG89 for L71 and 183GATATATGACCCTGTCTGT201 for L183) and the reverse complement oligonucleotides were linked by a 9-nucleotide hairpin-forming spacer (TTCAAGAGA). This forced the antisense SPIK oligonucleotides to fold back and form a double-stranded SPIK RNA complex with the sense oligonucleotides. Specific SPIK siRNA was generated after cleavage by ribonucleases in the RNA-induced silencing complex (RISC) (per the manufacturer's instructions; Ambion, Austin, TX). The HBV siRNA 2791 plasmid, which targets the region upstream of the HBV surface protein, was kindly provided by Nucleonics, Inc. (Horsham, PA). The HBV-expressing plasmid P13 contains a 1.3-mer HBV gene and has been described previously (23).
Transfection and induction of SPDCA.
For transfection, 105 cells were seeded in 60-mm dishes. After overnight incubation, 2 μg of plasmid was transfected with 6 μl FuGENE 6 as described by the manufacturer (Roche Diagnostics, Indianapolis, IN). Mock transfections, consisting of transfection of the relevant vector lacking the gene of interest, were used as transfection controls. The transfection efficiency was determined by transfection and expression of human green fluorescent protein (hGFP). After 3 days, cells were split into two daughter 60-mm dishes and cultured for an additional day. RNA was then isolated for Northern blot analysis from half of the cells, and SPDCA was induced in the other half by treatment with brefeldin A (BFA)-cycloheximide (CHX) (5 μg/ml-10 μg/ml). The pan-caspase inhibitor Z-VAD was also added (100 μM) to ensure inhibition of CDCA. Apoptosis was examined 24 h after treatment. For the silencing studies, cells were cotransfected with P13 plasmid and SPIK siRNA L71 or L183 or HBV siRNA 2791 plasmid following the transfection protocol described above.
Northern blotting.
For Northern blot analysis, total RNA was isolated from cells at 3 days posttransfection, using TRIzol reagent (Invitrogen, Carlsbad, CA). A 1% denaturing agarose gel was used to resolve 10 μg RNA, which was then transferred to a nylon membrane. SPIK-, HCV-, and HBV-specific probes were labeled with [32P]dCTP, using SPIK, HCV, and HBV template DNAs derived from PCR amplification. After hybridization, the bands were visualized using a phosphorimager, and accompanying graphical software was utilized to estimate the intensities of the bands. Equal sample loading was confirmed by ethidium bromide (EB)-stained rRNA in each experiment.
Western blotting.
The level of SPIK protein was examined by Western blotting, using an anti-SPIK (anti-SPINK) monoclonal antibody (Abcam, Cambridge, MA). Briefly, 15 μl of culture medium from the cells of interest was run in a 4 to 12% SDS-PAGE gel (Invitrogen, Carlsbad, CA). After transfer to a polyvinylidene difluoride (PVDF) membrane, SPIK protein was visualized by staining with a mouse monoclonal anti-SPIK antibody at a 1:1,000 dilution and then with a standard anti-mouse-horseradish peroxidase (HRP) secondary antibody at 1:7,500. An ECL Advance kit (GE Lifescience) was used to visualize the image, which was captured using a Gel Logic 1500 molecular imaging system and Kodak imaging software (Carestream Health Inc.).
Examination of apoptosis.
Apoptosis was examined by observing both characteristic morphology changes and annexin V-fluorescein isothiocyanate (annexin V-FITC) staining. For the annexin staining, BFA-CHX-Z-VAD-treated cells were washed with phosphate-buffered saline (PBS) and then incubated with annexin V-FITC in 100 μl of binding buffer (Trevigen Inc., Gaithersburg, MD). After incubating for 30 min, the cells were washed with PBS and then were visualized with a fluorescence microscope. For flow cytometry (fluorescence-activated cell sorting [FACS]) analysis, BFA-CHX-Z-VAD-treated cells were released by limited trypsin digestion and washed with PBS. The cells were resuspended in a 100-μl total volume of annexin and binding buffer per the manufacturer's instructions and then incubated at room temperature in the dark for 25 min (Biosource International, Camarillo, CA). For cells double stained with annexin V-FITC and propidium iodide (PI), 0.5 μg PI was added 3 min before the end of the annexin incubation. The cells were then resuspended in 400 μl binding buffer, and apoptosis was analyzed using a Guava EasyCyte Plus system (Guava Technologies, Hayward, CA). Compensation was done to minimize the overlapping wavelengths of FITC and PI. For FACS data presented as histograms, cells were gated on only singly annexin V-positive cells to avoid including possible necrotic, PI-positive cells.
Induction of CDCA and caspase 3 analysis.
CDCA was induced by treatment with etoposide and staurosporine. Cells were treated with 400 μM etoposide for 24 to 48 h or with 1 μM staurosporine for 24 h. As a control, cells were also treated with the SPDCA inducers BFA and CHX as before. Z-VAD was added to the BFA-CHX treatment to completely block caspase activity during SPDCA induction. Cell morphology changes were examined, and caspase 3 activity was analyzed using a BD ApoAlert caspase colorimetric assay kit (BioVision, Mountain View, CA). The results were read using an SLT Rainbow microplate reader at 405 nm.
Suppression of HCV replication.
In order to suppress HCV replication, HCV inhibitors such as HCV polymerase inhibitor 888 (a generous gift from B. Gu, Novartis, Shanghai, China) and ribavirin (Toronto Research Chemicals Inc., Toronto, Canada) were incubated with the cells for 2 days. After treatment, total RNA was isolated for Northern blot analysis. For alpha interferon treatment, cells were incubated for 24 h with 500 U/ml alpha interferon, and total RNA was isolated for Northern blot analysis.
Cell viability.
G54 cells and control cells were seeded in a 96-well plate. The cells were then incubated with increasing doses of ribavirin. After 2 days, cell proliferation was examined using a WST cell viability kit (Roche, Indianapolis, IN) per the manufacturer's instructions. Optical density was measured at 440 nm, using a microplate reader.
RESULTS
HCV replication upregulates SPIK expression, resulting in resistance to SPDCA.
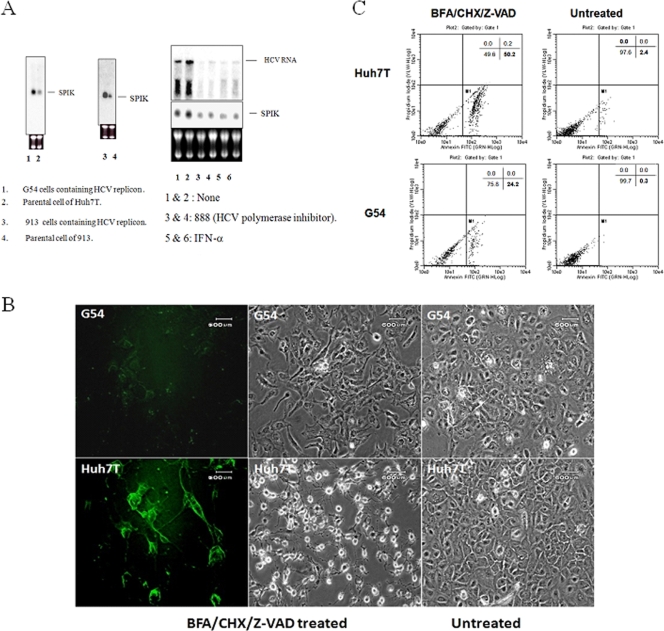
In order to study the relationship between HCV replication and SPIK expression, Northern blot analysis was done to compare SPIK RNA transcript levels in the G54 cell line, which contains the HCV genome as a replicon and constitutively produces HCV, and its parental cell line Huh7T, which is susceptible to serine protease-dependent cell apoptosis (22). Northern blotting showed notably more SPIK RNA in the G54 cells than in the parental Huh7T cells (Fig. 1A, left panel, lanes 1 and 2). More SPIK RNA (>3-fold) was found in G54 cells than in parental cells. To control for possible differences between replicon cells from different sources, another HCV replicon was examined for SPIK expression along with its parental cell. As expected, SPIK RNA levels were also substantially increased in the HCV replicon-expressing 913 cells (14), showing a nearly threefold increase in SPIK compared to the parental cells (Fig. 1A, left panel, lanes 3 and 4). These results suggest that HCV replication upregulates SPIK expression.
FIG. 1.
HCV replication upregulates SPIK expression, resulting in cell resistance to SPDCA. (A) Northern blot showing SPIK and HCV RNA. Total RNA was isolated from G54 or 913 HCV replicon cells and their parental cells. Ten micrograms of RNA was resolved in a 1% denaturing agarose gel and transferred to a nylon membrane, followed by hybridization with probes specific for SPIK and HCV. For suppression of HCV replication, G54 cells were pretreated with the HCV polymerase inhibitor 888 or with alpha interferon, and Northern blotting was then performed. Ethidium bromide (EB)-stained rRNA was used as a loading control in each experiment. (B) Apoptosis was induced in G54 cells and parental Huh7T cells by treatment with BFA-CHX-Z-VAD. Cell apoptosis was examined by observation of morphology changes and annexin V-FITC staining with a fluorescence microscope. (C) Apoptotic cells were quantified by flow cytometry after double staining with annexin V-FITC and propidium iodide (PI). Cells in the bottom-right quadrant represent apoptotic cells stained by annexin V-FITC.
Further evidence of a role of HCV replication in elevation of SPIK transcript levels was shown by inhibiting HCV replication by using either HCV polymerase inhibitor 888 or alpha interferon, two HCV replication inhibitors. In this case, the effect of HCV replication on SPIK can be demonstrated even more clearly. Northern blot analysis showed that treatment of G54 cells with 888 or alpha interferon caused a >3-fold reduction in HCV genomic RNA and, at the same time, that SPIK RNA was also reduced, although the reduction was not as significant as that of HCV RNA (Fig. 1A, right panel).
After showing that HCV replication is associated with increased SPIK expression, apoptosis studies were performed to demonstrate that this increase in SPIK levels leads to cell resistance to apoptosis. Although it has previously been shown that treatment with BFA-CHX specifically induces SPDCA (6), the pan-caspase inhibitor Z-VAD was included in the treatment to completely exclude the influence of the caspases. Figure 1B shows that treatment with BFA-CHX-Z-VAD induced apoptosis, which is characterized by cell shrinkage and membrane blebbing, in nearly all of the parental Huh7T cells. The G54 cells, however, showed only slight morphology changes (Fig. 1B, phase-contrast images), which implied a decreased induction of apoptosis. Apoptosis was also analyzed by annexin staining, which identifies cell surface changes that occur in the early stages of apoptosis. After incubation of BFA-CHX-Z-VAD-treated cells with annexin V-FITC, strong fluorescence was visible only in Huh7T cells, while very few G54 cells showed positive staining (Fig. 1B, annexin staining). This weak annexin staining indicates that G54 cells are more resistant to BFA-CHX-Z-VAD-induced SPDCA. Quantification of annexin staining by flow cytometry suggested that 50% of Huh7T cells underwent apoptosis, while only 24% of the G54 cells were apoptotic after treatment with BFA-CHX-Z-VAD (Fig. 1C). In contrast, there was no significant difference between these two cell types under untreated conditions (Fig. 1C, untreated panels). Together, these data strongly support the hypothesis that HCV replication increases levels of SPIK, resulting in HCV-expressing cells being more resistant to SPDCA.
SPIK does not affect CDCA induction in HCV replicon cells.
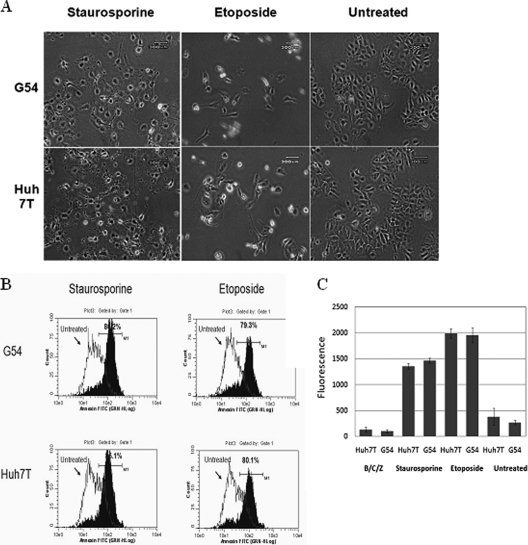
Because SPIK is an apoptosis inhibitor, the effect of increased SPIK expression in G54 cells on the caspase-dependent cell apoptosis pathway needed to be determined as well. To test for inhibition of CDCA, G54 and Huh7T cells were treated with etoposide or staurosporine, two frequently used CDCA inducers. Obvious changes in cell morphology, indicative of apoptosis, were seen in all cell lines after treatment (Fig. 2A). Flow cytometry data suggested that approximately 79% of G54 cells and 80% of Huh7T cells showed positive annexin staining after treatment with etoposide and that 80% of G54 cells and 86% of Huh7T cells were positive after treatment with staurosporine (Fig. 2B). This suggests that SPIK expression has no inhibitory effects upon CDCA, because apoptosis occurs similarly in both cell lines, regardless of SPIK expression.
FIG. 2.
HCV replication does not suppress CDCA. CDCA was induced in both HCV-expressing G54 cells and the parental cells by treatment with the CDCA inducer staurosporine or etoposide. (A). Apoptosis, represented by cell morphology changes, was examined with a phase-contrast microscope. (B) Apoptotic cells were quantified by flow cytometry after being stained with annexin V-FITC. Shifting of the peak toward increased fluorescence (toward the right) represents apoptotic cells. The percentage of apoptotic cells is indicated. (C) The activity of caspase 3, a marker to determine CDCA activation, was assessed using a caspase 3 detection kit. CDCA was induced in G54 cells and parental Huh7T cells by treatment with staurosporine or etoposide. Caspase 3 activity was then detected with a fluorescence reader.
Caspase 3 activity was also used as a marker to determine CDCA induction. After treatment of cells with etoposide or staurosporine, caspase 3 activity was analyzed using a caspase 3 detection kit (Biovision, Mountain View, CA). Figure 2C shows that even though G54 cells produced nearly threefold more SPIK than did Huh7T cells (Fig. 1A), the induction of caspase 3 via etoposide or staurosporine treatment was similar in both cell lines. In contrast, there was little or no caspase 3 activity in untreated cells as well as in cells treated with the SPDCA inducers BFA, CHX, and Z-VAD (Fig. 2C). The lack of caspase 3 activity in the cells treated with BFA-CHX-Z-VAD suggests that apoptosis induced by BFA-CHX is independent of caspase activity and that CDCA in HCV-infected cells would be unaffected by increased levels of SPIK.
HBV replication upregulates SPIK expression, resulting in resistance to SPDCA.
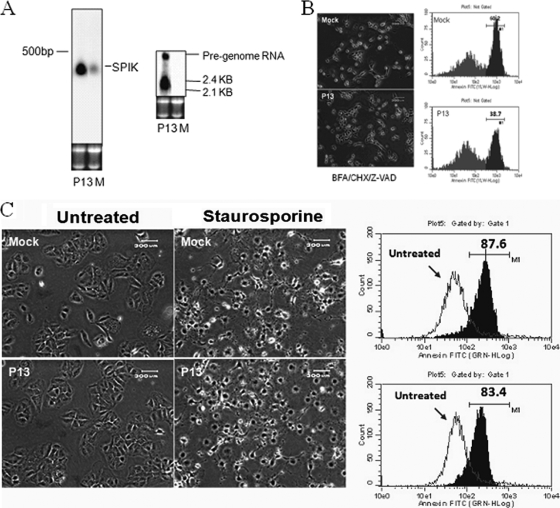
Similar experiments to those performed with HCV were performed to determine if upregulation of SPIK is also associated with HBV replication. Because HepG2.2.15 cells, a stable cell line frequently used for studying HBV replication, have a very different proliferation profile from that of the parental cell line, HepG2, we decided to use transfection for the HBV studies. After transfection of Huh7T cells with plasmid P13, which contains the entire HBV genome, Northern blot analysis showed HBV pregenomic RNA and 2.1/2.4-kb RNA, suggesting replication of HBV in these cells (Fig. 3A). Interestingly, an increase in SPIK expression was also clearly visible in P13-transfected cells. Compared to that of mock-transfected cells, the SPIK RNA level was increased nearly threefold after transfection of P13 (Fig. 3A). This suggests that like the case for HCV, HBV replication can upregulate SPIK expression.
FIG. 3.
HBV replication upregulates SPIK, resulting in resistance to SPCDA but not to CDCA. (A) Huh7T cells were transfected with P13, which contains the entire HBV genome. Vector lacking the HBV genome was transfected as a control (mock transfection [lanes M]). HBV replication markers, i.e., pregenomic RNA and 2.1/2.4-kb RNA, and SPIK RNA were examined by Northern blotting with HBV- and SPIK-specific probes. (B) SPDCA was induced in P13-transfected cells and control cells by treatment with BFA-CHX-Z-VAD. Apoptosis was examined by morphology changes and flow cytometry after annexin V-FITC staining. Apoptotic cells are shown by a peak shift toward higher fluorescence. The percentage of apoptotic cells is indicated. (C). CDCA was induced in P13-transfected cells and control cells by treatment with staurosporine. Apoptosis was examined by morphology changes (left panels) or flow cytometry after annexin staining (right panels). A shift of the peak toward higher fluorescence indicates increased apoptosis (right panels). The percentage of apoptotic cells is indicated.
To confirm the role of HBV-associated SPIK expression in SPDCA, a series of apoptosis experiments were conducted, similar to those done for HCV. Resistance to apoptosis was demonstrated through previously described apoptosis-associated morphology changes in P13-transfected cells after treatment with BFA-CHX-Z-VAD (Fig. 3B). Flow cytometry data indicated that 39% of transfected cells underwent apoptosis, while 60% of the nontransfected cells underwent apoptosis (Fig. 3B). Double staining with anti-HBsAg and annexin suggested that most of the HBsAg-positive cells were negative for annexin staining. This implies that the cells positive for HBV were not apoptotic and that the apoptotic cells were not expressing HBV (see Fig. S1 in the supplemental material). Together, these results suggest that HBV replication can upregulate SPIK expression, resulting in the resistance of HBV cells to SPDCA. As was the case with the HCV replicon cells, the increased expression of SPIK did not prevent CDCA in HBV-transfected cells. The transfection of P13 did not suppress cell apoptotic death induced by the CDCA inducer staurosporine (Fig. 3C).
Suppression of overexpressed SPIK in G54 cells restores sensitivity to SPDCA.
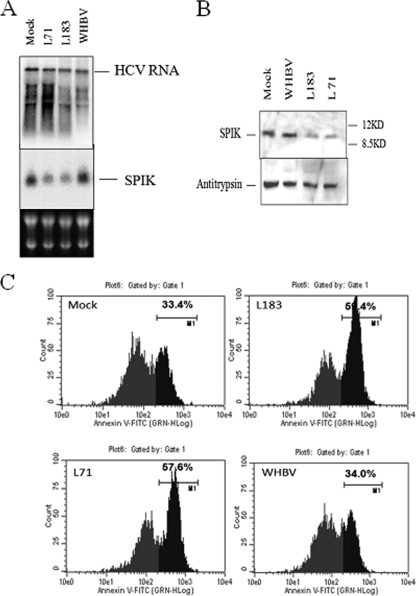
Since HBV and HCV replication upregulates SPIK, leading to suppression of SPDCA, it was of interest to determine if suppression of the overexpressed SPIK in these cells restored sensitivity to SPDCA. For this purpose, two SPIK siRNAs, L71 and L183, were constructed. Northern blot analysis was done to confirm successful suppression of SPIK in the G54 cells by the L71 and L183 siRNAs. Figure 4A shows that transfection with either SPIK siRNA reduced SPIK expression two- to threefold compared to mock transfection with empty vector. As expected, silencing of SPIK RNA expression resulted in a decrease in expression of SPIK protein. Western blot data suggested that transfection with SPIK siRNAs L71 and L183 reduced the SPIK protein expression two- to threefold (Fig. 4B). In addition, the silencing effect of both SPIK siRNAs was specific, because siRNA for woodchuck hepatitis B virus (WHBV) did not affect SPIK expression at either the mRNA level or the protein level (Fig. 4A and B). Also, the specificity of the silencing by L71 and L183 was seen in the fact that these siRNAs did not affect HCV RNA expression (Fig. 4A). These findings are in line with our previously published data in which we demonstrated that resistance to SPDCA was directly related to the SPIK protein level (22). In addition, reduction of SPIK protein expression restored sensitivity of the cells to SPDCA, as it was seen that transfection with L71 and L183 dramatically increased the level of apoptotic death in G54 cells after treatment with BFA-CHX-Z-VAD. Flow cytometry data showed that while 57% of G54 cells transfected with L71 and 59% of G54 cells transfected with L183 underwent apoptosis after BFA-CHX-Z-VAD treatment, only 33% of mock-transfected cells and 34% of cells transfected with the unrelated WHBV siRNA were apoptotic (Fig. 4B). Since it has already been shown that silencing does not affect HCV expression (Fig. 4A), the results here suggest that HCV replication itself does not prevent cell apoptotic death. The data do, however, suggest that SPIK, which is upregulated by HCV replication, is responsible for resistance to SPDCA. It also suggests that reduction of SPIK expression can restore cell killing through the serine protease-dependent apoptotic pathway.
FIG. 4.
Silencing SPIK in G54 cells can restore sensitivity to SPDCA. G54 cells were transfected with SPIK siRNAs L71 and L183 and with control WHBV siRNA. Empty siRNA vector was transfected as a control (mock transfection). (A) HCV and SPIK mRNAs were determined by Northern blotting as previously described. Equal loading was confirmed by ethidium bromide (EB) staining of rRNA. (B). SPIK protein levels were determined by Western blotting with anti-SPIK (anti-SPINK) monoclonal antibody. Alpha-1-antitrypsin was used as a loading control. (C) Apoptosis was induced by treatment of cells with BFA-CHX-Z-VAD after transfection. Apoptosis of cells transfected with siRNA was determined by flow cytometry after staining with annexin V-FITC. Apoptotic cells are represented by peaks with higher fluorescence. The percentage of apoptotic cells is indicated.
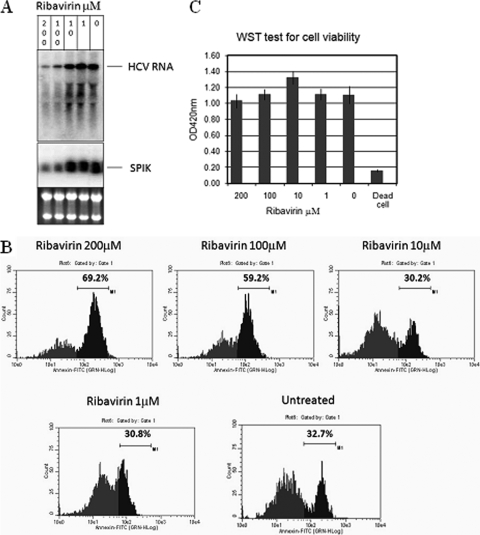
To further confirm our hypothesis, G54 cells were treated with ribavirin, an HCV suppressor which is used as a drug against HCV infection (17). Treatment with ribavirin resulted in a dose-dependent decrease in HCV replication in G54 cells (Fig. 5A). Since SPIK induction is associated with HCV replication (Fig. 3), treatment with ribavirin also reduced SPIK expression (Fig. 5A), as would be expected. At higher doses of ribavirin, clear reductions of HCV and SPIK expression were observed, and importantly, restoration of apoptotic death was seen at the same doses after treatment with BFA-CHX-Z-VAD. Flow cytometry data showed that the cells undergoing apoptosis increased from approximately 30% to 60% and 69% with 100 μM and 200 μM ribavirin treatments, respectively (Fig. 5B). To show that cell death seen at these high doses was not due to deleterious effects of ribavirin directly on the cells, G54 cells were treated with increasing doses of ribavirin and a WST test for cell viability was done. These results showed that even at doses as high as 200 μM, ribavirin alone was not sufficient to induce cell death (Fig. 5C), and the increase of SPDCA observed after treatment with BFA-CHX-Z-VAD was caused by a decrease in SPIK expression. To prove that ribavirin was not having a direct effect on SPIK, S2-3 cells, our SPIK-expressing stable cell line (22), were treated with ribavirin. No effect of ribavirin treatment was seen on SPIK mRNA levels or induction of apoptosis (see Fig. S2 in the supplemental material).
FIG. 5.
Suppression of HCV replication restores sensitivity to SPDCA. G54 cells were treated with different doses of the HCV inhibitor ribavirin. (A) Suppression of HCV replication and SPIK expression was examined by Northern blotting, using HCV- and SPIK-specific probes as previously described. (B) Resistance of G54 cells to SPDCA was quantified by flow cytometry after treatment with BFA-CHX-Z-VAD. Apoptotic cells are represented by peaks with higher fluorescence. The percentage of apoptotic cells is indicated. (C) The effect of ribavirin treatment on cell viability was examined by a WST test kit after treatment of G54 cells with increasing concentrations of ribavirin.
Suppression of overexpressed SPIK in HBV-expressing cells can also restore sensitivity to SPDCA.
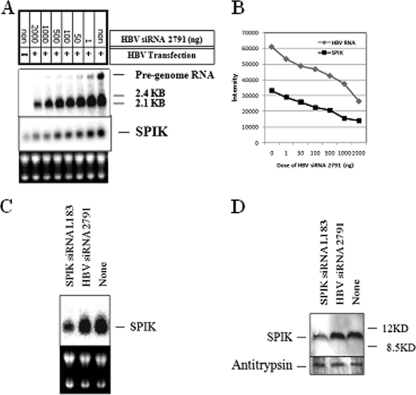
To confirm that suppression of SPIK in HBV-expressing cells restores apoptotic death, a series of experiments were done using cotransfections. Cotransfection of P13 and HBV siRNA 2791 reduced HBV expression in a dose-dependent manner, which was shown by Northern blotting (Fig. 6A). The HBV replication markers, i.e., pregenomic RNA and 2.1/2.4-kb RNA, were both reduced after cotransfection with siRNA 2791. Although siRNA 2791 directly targets the region upstream of the coding region of the surface protein, the degradation of 2.1/2.4-kb RNA was observed. This is a phenomenon that has previously been reported by other groups using siRNAs that do not directly target surface protein (15, 26, 35, 44). As expected, cotransfection of siRNA 2791 with P13 also reduced SPIK expression accordingly (Fig. 6B). The HBV siRNA 2791 did not have a direct effect on SPIK, however, as up to 2 μg of this siRNA did not suppress either SPIK RNA or protein expression in the SPIK-expressing stable cell line S2-3, while reductions of both SPIK RNA and protein were observed in these cells after transfection of the SPIK siRNA L183 (Fig. 6C and D). This suggests that the reduction of SPIK seen in the cotransfections was due to the suppression of HBV expression, not to direct silencing of SPIK by the HBV-specific siRNA 2791. As expected, SPIK siRNA did not suppress HBV replication. Northern blot analysis showed that cotransfection of up to 2 μg L183 did not reduce HBV RNA expression, while 2 μg HBV 2791 clearly suppressed HBV transcription (see Fig. S3 in the supplemental material). This was also confirmed by Southern blot detection of HBV DNA and detection of secreted HBsAg in culture medium after transfection. Cotransfection of L183 with P13 did not reduce either HBV DNA expression or HBV surface protein expression (see Fig. S3 in the supplemental material).
FIG. 6.
Suppression of HBV replication restores sensitivity to SPDCA. Huh7T cells were transfected with the HBV plasmid P13. To silence HBV replication, different doses of HBV siRNA 2791 were cotransfected with P13. (A) Northern blotting was used to determine the amount of HBV replication as well as SPIK expression in transfected cells, using HBV- and SPIK-specific probes. (B) Quantification of HBV RNA versus SPIK RNA in cotransfected Huh7T cells shows the relationship between HBV RNA level and SPIK level. (C and D) HBV siRNA 2791 did not silence SPIK. The cell line S2-3, which stably expresses SPIK (22), was transfected with either SPIK siRNA L183 (1 μg) or HBV siRNA 2791 (2 μg). The expression of SPIK was determined by Northern blotting (C) and Western blotting (D).
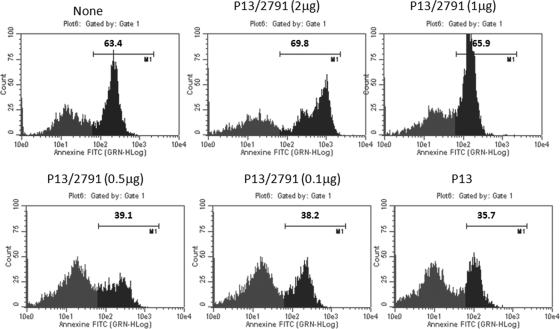
The suppression of SPIK by cotransfection of HBV 2791 in P13-transfected cells was sufficient to restore susceptibility to SPDCA in these cells once apoptosis was induced by treatment of the cells with BFA-CHX-Z-VAD. Transfection with P13 resulted in the reduction of apoptotic cells from 63% to 36% after BFA-CHX-Z-VAD treatment (Fig. 7, mock transfection and P13 panels). Cotransfection with siRNA 2791, however, reduced HBV expression gradually and proportionally increased apoptotic death after BFA-CHX-Z-VAD treatment, until SPIK was nearly at background levels and apoptotic death nearly returned to the level for mock-transfected cells (Fig. 6 and 7).
FIG. 7.
HBV-transfected cells show a siRNA dose-dependent increase in sensitivity to SPDCA. Huh7T cells were transfected with P13 and cotransfected with increasing doses of the HBV siRNA 2791. Apoptosis was quantified by flow cytometry analysis of annexin V staining after treatment of cells with BFA-CHX-Z-VAD. Apoptotic cells are represented by peaks with higher fluorescence. The percentage of apoptotic cells is indicated.
DISCUSSION
Although the results which we have presented here can only suggest that HBV and HCV replication upregulates SPIK expression, they are consistent with the observation that SPIK is overexpressed in HBV/HCV-infected livers (32). More importantly, our results show that this upregulation of SPIK results in resistance to SPDCA. As stated earlier, SPDCA is a recently described pathway of apoptosis in which serine proteases, not caspases, are the critical players. Interestingly, apoptosis induced by granzyme A (GzmA) has been shown to be independent of caspase activity and was suppressed by the serine protease inhibitor DCI (3,4-dichloroisocoumarin) (2, 25, 37). GzmA is a serine protease secreted by CTLs and natural killer cells (NK cells) to induce the apoptotic death of target cells as part of the immune response. It plays an important role in the clearance of virus-infected cells and cancer precursor/cancer cells, preventing the expansion of malignant cells which would otherwise develop into tumors (20, 33). Since GzmA is a serine protease, it is possible that the serine protease inhibitor SPIK, which can suppress serine protease-dependent cell apoptosis, could inhibit GzmA activity, consequently blocking GzmA-induced cell apoptosis. This hypothesis is supported by an observation which showed that rat SPIK can directly bind GzmA and inhibit its ability to hydrolyze substrates such as N-α-benzyloxycarbonyl-l-lysine thiobenzyl ester (BLT) (42). Both HBV and HCV may render their host cells insensitive to CTL and NK cell responses in patients (13). Based on these findings, we hypothesize that the escape of HBV/HCV-infected cells from CTL- and NK cell-associated immune surveillance, such as cell clearance via GzmA-mediated apoptosis, may be due to an elevation of SPIK levels in these cells. Because of the inability of HBV- and HCV-infected patients to clear infected cells due to increased SPIK levels, the virus is able to persistently replicate in these cells, leading to chronic hepatitis and, ultimately, cancer. This may be the common denominator for the similarities in the pathogeneses of these two distinctly different viruses. Furthermore, the additional hypothesis that SPIK may be involved in the development of HBV/HCV-associated HCC is supported by multiple observations. First, the level of SPIK in patients has been found to correlate with the progression of HCC (34). Moreover, high levels of SPIK are closely related to early recurrence of HCC in patients after surgical resection (19). Because recurrence of cancer often implies an inability to clear lingering oncogenic cells by the immune system, early recurrence of HCC in patients with high levels of SPIK would fit the hypothesis that overexpression of SPIK interferes with the elimination of lingering oncogenic cells by suppressing immune-mediated apoptosis. The expansion of these residual oncogenic cells could then lead to cancer recurrence. Since SPIK is also elevated in other cancers (16, 19, 24, 31, 40, 41), it is possible that this could be a common process of cancer formation.
How HBV/HCV triggers the overexpression of SPIK is still unclear. Because our results suggest that upregulation of SPIK is directly related to HBV/HCV replication, the specific mechanism for viral upregulation of SPIK is a topic currently under investigation. However, considering that the in vivo mechanisms may differ from our in vitro system, we cannot confidently include or exclude the role of upregulation of SPIK in actual virus infection. Studies with this in mind, including the use of infection systems such as the established infection systems for HCV, are currently being pursued.
It is interesting that recent studies show that SPIK can be activated as a reactant during hepatitis or liver inflammation (30). For example, SPIK was activated in rat liver cells to counter turpentine-induced liver inflammation (43). It is possible, therefore, that in chronic HBV and HCV infections, persistent liver inflammation due to viral replication triggers the overexpression of SPIK, which in turn allows virally infected cells to survive apoptotic death, and that a positive feedback loop thus develops. This is supported by the observation that SPIK is activated in response to inflammatory cytokines during human viral hepatitis (10). The exact cytokines involved in activation of SPIK, however, are still unknown. It was reported that an area upstream of the SPIK gene contained a binding site for interleukin 6 (IL-6), which might suggest a possible role of IL-6 in triggering SPIK expression (45). Alternatively, components of HBV or HCV may be directly responsible for activation of SPIK, and studies to determine these components, using HBV and HCV proteins, are currently under way in our laboratory.
Supplementary Material
Acknowledgments
This work was supported by an appropriation from the Commonwealth of Pennsylvania, by the Hepatitis B Foundation, and by the National Cancer Institute, NIH.
We thank Baohua Gu (Novartis, Shanghai, China) and Tianlun Zhou (Nucleonic Inc., Horsham, PA [currently with Novartis, Shanghai, China]) for providing the G54 and Huh7T cell lines and Jutao Guo (Drexel University, Doylestown, PA) for the 913 cells. We thank Wolfram Gerlich (Giessen, Germany) for a critical reading of the manuscript.
Footnotes
Published ahead of print on 28 October 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Abate, A., and H. Schroder. 1998. Protease inhibitors protect macrophages from lipopolysaccharide-induced cytotoxicity: possible role for NF-[kappa]B. Life Sci. 62:1081-1088. [DOI] [PubMed] [Google Scholar]
- 2.Beresford, P. J., D. Zhang, D. Y. Oh, Z. Fan, E. L. Greer, M. L. Russo, M. Jaju, and J. Lieberman. 2001. Granzyme A activates an endoplasmic reticulum-associated caspase-independent nuclease to induce single-stranded DNA nicks. J. Biol. Chem. 276:43285-43293. [DOI] [PubMed] [Google Scholar]
- 3.Chisari, F. V. 1997. Cytotoxic T cells and viral hepatitis. J. Clin. Invest. 99:1472-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chisari, F. V., and C. Ferrari. 1995. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 13:29-60. [DOI] [PubMed] [Google Scholar]
- 5.de Bruin, E. C., D. Meersma, J. de Wilde, I. den Otter, E. M. Schipper, J. P. Medema, and L. T. Peltenburg. 2003. A serine protease is involved in the initiation of DNA damage-induced apoptosis. Cell Death Differ. 10:1204-1212. [DOI] [PubMed] [Google Scholar]
- 6.Egger, L., J. Schneider, C. Rheme, M. Tapernoux, J. Hacki, and C. Borner. 2003. Serine proteases mediate apoptosis-like cell death and phagocytosis under caspase-inhibiting conditions. Cell Death Differ. 10:1188-1203. [DOI] [PubMed] [Google Scholar]
- 7.Giannini, C., and C. Brechot. 2003. Hepatitis C virus biology. Cell Death Differ. 10(Suppl. 1):S27-S38. [DOI] [PubMed] [Google Scholar]
- 8.Graf, R., and D. Bimmler. 2006. Biochemistry and biology of SPINK-PSTI and monitor peptide. Endocrinol. Metab. Clin. N. Am. 35:333-343. [DOI] [PubMed] [Google Scholar]
- 9.Greene, L. J. 1975. Pancreatic exocrine secretory proteins. J. Surg. Oncol. 7:151-154. [DOI] [PubMed] [Google Scholar]
- 10.Greene, L. J., M. H. Pubols, and D. C. Bartelt. 1976. Human pancreatic secretory trypsin inhibitor. Methods Enzymol. 45:813-825. [DOI] [PubMed] [Google Scholar]
- 11.Gu, B., A. T. Gates, O. Isken, S. E. Behrens, and R. T. Sarisky. 2003. Replication studies using genotype 1a subgenomic hepatitis C virus replicons. J. Virol. 77:5352-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guicciardi, M. E., and G. J. Gores. 2005. Apoptosis: a mechanism of acute and chronic liver injury. Gut 54:1024-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidotti, L. G., and F. V. Chisari. 2006. Immunobiology and pathogenesis of viral hepatitis. Annu. Rev. Pathol. 1:23-61. [DOI] [PubMed] [Google Scholar]
- 14.Guo, J.-T., V. V. Bichko, and C. Seeger. 2001. Effect of alpha interferon on the hepatitis C virus replicon. J. Virol. 75:8516-8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo, Y., H. Guo, L. Zhang, H. Xie, X. Zhao, F. Wang, Z. Li, Y. Wang, S. Ma, J. Tao, W. Wang, Y. Zhou, W. Yang, and J. Cheng. 2005. Genomic analysis of anti-hepatitis B virus (HBV) activity by small interfering RNA and lamivudine in stable HBV-producing cells. J. Virol. 79:14392-14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higashiyama, M., T. Monden, N. Tomita, M. Murotani, Y. Kawasaki, H. Morimoto, A. Murata, T. Shimano, M. Ogawa, and T. Mori. 1990. Expression of pancreatic secretory trypsin inhibitor (PSTI) in colorectal cancer. Br. J. Cancer 62:954-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmann, W. P., E. Herrmann, C. Sarrazin, and S. Zeuzem. 2008. Ribavirin mode of action in chronic hepatitis C: from clinical use back to molecular mechanisms. Liver Int. 28:1332-1343. [DOI] [PubMed] [Google Scholar]
- 18.Kerr, J. F., C. M. Winterford, and B. V. Harmon. 1994. Apoptosis. Its significance in cancer and cancer therapy. Cancer 73:2013-2026. [DOI] [PubMed] [Google Scholar]
- 19.Lee, Y. C., H. W. Pan, S. Y. Peng, P. L. Lai, W. S. Kuo, Y. H. Ou, and H. C. Hsu. 2007. Overexpression of tumour-associated trypsin inhibitor (TATI) enhances tumour growth and is associated with portal vein invasion, early recurrence and a stage-independent prognostic factor of hepatocellular carcinoma. Eur. J. Cancer 43:736-744. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman, J. 2003. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat. Rev. Immunol. 3:361-370. [DOI] [PubMed] [Google Scholar]
- 21.Lu, X., and T. Block. 2004. Study of the early steps of the hepatitis B virus life cycle. Int. J. Med. Sci. 1:21-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu, X., J. Lamontagne, F. Lu, and T. Block. 2008. Tumor-associated protein SPIK/TATI suppresses serine protease dependent cell apoptosis. Apoptosis 13:483-494. [DOI] [PubMed] [Google Scholar]
- 23.Lu, X., Y. Lu, R. Geschwindt, R. A. Dwek, and T. M. Block. 2001. Hepatitis B virus MHBs antigen is selectively sensitive to glucosidase-mediated processing in the endoplasmic reticulum. DNA Cell Biol. 20:647-656. [DOI] [PubMed] [Google Scholar]
- 24.Lukkonen, A., S. Lintula, K. von Boguslawski, O. Carpen, B. Ljungberg, G. Landberg, and U. H. Stenman. 1999. Tumor-associated trypsin inhibitor in normal and malignant renal tissue and in serum of renal-cell carcinoma patients. Int. J. Cancer 83:486-490. [DOI] [PubMed] [Google Scholar]
- 25.Mahrus, S., and C. S. Craik. 2005. Selective chemical functional probes of granzymes A and B reveal granzyme B is a major effector of natural killer cell-mediated lysis of target cells. Chem. Biol. 12:567-577. [DOI] [PubMed] [Google Scholar]
- 26.McCaffrey, A. P., H. Nakai, K. Pandey, Z. Huang, F. H. Salazar, H. Xu, S. F. Wieland, P. L. Marion, and M. A. Kay. 2003. Inhibition of hepatitis B virus in mice by RNA interference. Nat. Biotechnol. 21:639-644. [DOI] [PubMed] [Google Scholar]
- 27.Mullbacher, A., K. Ebnet, R. V. Blanden, R. T. Hla, T. Stehle, C. Museteanu, and M. M. Simon. 1996. Granzyme A is critical for recovery of mice from infection with the natural cytopathic viral pathogen, ectromelia. Proc. Natl. Acad. Sci. USA 93:5783-5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholson, D. W., and N. A. Thornberry. 1997. Caspases: killer proteases. Trends Biochem. Sci. 22:299-306. [DOI] [PubMed] [Google Scholar]
- 29.Nunez, G., M. A. Benedict, Y. Hu, and N. Inohara. 1998. Caspases: the proteases of the apoptotic pathway. Oncogene 17:3237-3245. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa, M., T. Shibata, T. Niinobu, K. Uda, N. Takata, and T. Mori. 1988. Serum pancreatic secretory trypsin inhibitor (PSTI) in patients with inflammatory diseases. Adv. Exp. Med. Biol. 240:505-508. [DOI] [PubMed] [Google Scholar]
- 31.Ohmachi, Y., A. Murata, N. Matsuura, T. Yasuda, T. Yasuda, M. Monden, T. Mori, M. Ogawa, and K. Matsubara. 1993. Specific expression of the pancreatic-secretory-trypsin-inhibitor (PSTI) gene in hepatocellular carcinoma. Int. J. Cancer 55:728-734. [DOI] [PubMed] [Google Scholar]
- 32.Ohmachi, Y., A. Murata, T. Yasuda, K. Kitagawa, S. Yamamoto, M. Monden, T. Mori, N. Matsuura, and K. Matsubara. 1994. Expression of the pancreatic secretory trypsin inhibitor gene in the liver infected with hepatitis B virus. J. Hepatol. 21:1012-1016. [DOI] [PubMed] [Google Scholar]
- 33.Pardo, J., A. Bosque, R. Brehm, R. Wallich, J. Naval, A. Mullbacher, A. Anel, and M. M. Simon. 2004. Apoptotic pathways are selectively activated by granzyme A and/or granzyme B in CTL-mediated target cell lysis. J. Cell Biol. 167:457-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehermann, B., and M. Nascimbeni. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5:215-229. [DOI] [PubMed] [Google Scholar]
- 35.Ren, G.-L., X.-F. Bai, Y. Zhang, H.-M. Chen, C.-X. Huang, P.-Z. Wang, G.-Y. Li, Y. Zhang, and J.-Q. Lian. 2005. Stable inhibition of hepatitis B virus expression and replication by expressed siRNA. Biochem. Biophys. Res. Commun. 335:1051-1059. [DOI] [PubMed] [Google Scholar]
- 36.Roger, W. 2006. Global challenges in liver disease. Hepatology 44:521-526. [DOI] [PubMed] [Google Scholar]
- 37.Shresta, S., T. A. Graubert, D. A. Thomas, S. Z. Raptis, and T. J. Ley. 1999. Granzyme A initiates an alternative pathway for granule-mediated apoptosis. Immunity 10:595-605. [DOI] [PubMed] [Google Scholar]
- 38.Stenman, U.-H. 2002. Tumor-associated trypsin inhibitor. Clin. Chem. 48:1206-1209. [PubMed] [Google Scholar]
- 39.Thorburn, J., L. M. Bender, M. J. Morgan, and A. Thorburn. 2003. Caspase- and serine protease-dependent apoptosis by the death domain of FADD in normal epithelial cells. Mol. Biol. Cell 14:67-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomita, N., S. Doi, M. Higashiyama, H. Morimoto, M. Murotani, Y. Kawasaki, T. Monden, T. Shimano, A. Horii, H. Yokouchi, et al. 1990. Expression of pancreatic secretory trypsin inhibitor gene in human colorectal tumor. Cancer 66:2144-2149. [DOI] [PubMed] [Google Scholar]
- 41.Tonouchi, A., M. Ohtsuka, H. Ito, F. Kimura, H. Shimizu, M. Kato, Y. Nimura, K. Iwase, T. Hiwasa, N. Seki, M. Takiguchi, and M. Miyazaki. 2006. Relationship between pancreatic secretory trypsin inhibitor and early recurrence of intrahepatic cholangiocarcinoma following surgical resection. Am. J. Gastroenterol. 101:1601-1610. [DOI] [PubMed] [Google Scholar]
- 42.Tsuzuki, S., Y. Kokado, S. Satomi, Y. Yamasaki, H. Hirayasu, T. Iwanaga, and T. Fushiki. 2003. Purification and identification of a binding protein for pancreatic secretory trypsin inhibitor: a novel role of the inhibitor as an anti-granzyme A. Biochem. J. 372:227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uda, K.-I., A. Murata, J.-I. Nishijima, S. Doi, N. Tomita, M. Ogawa, and T. Mori. 1994. Elevation of circulating monitor peptide/pancreatic secretory trypsin inhibitor-I (PSTI-61) after turpentine-induced inflammation in rats: hepatocytes produce it as an acute phase reactant. J. Surg. Res. 57:563-568. [DOI] [PubMed] [Google Scholar]
- 44.Xiao-Nan Zhang, W. X., J.-D. Wang, Y.-W. Hu, L. Xiang, and Z.-H. Yuan. 2004. siRNA-mediated inhibition of HBV replication and expression. World J. Gastroenterol. 10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasuda, T., M. Ogawa, A. Murata, Y. Ohmachi, T. Yasuda, T. Mori, and K. Matsubara. 1993. Identification of the IL-6-responsive element in an acute-phase-responsive human pancreatic secretory trypsin inhibitor-encoding gene. Gene 131:275-280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.