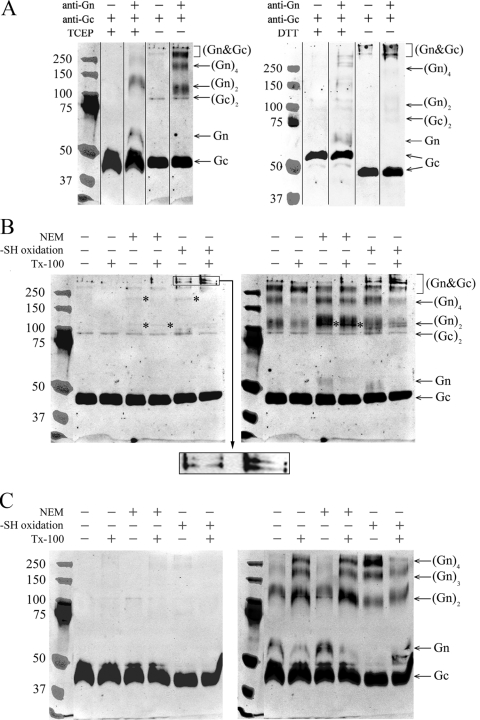
FIG. 4.
Glycoprotein complexes of TULV formed under reducing and nonreducing conditions and under separation by SDS-PAGE (samples boiled in panels A, B, and C). (A) Glycoprotein mobilities of TULV pelleted through sucrose cushion, treated with the reductant (TCEP or DTT) or left untreated, and separated by SDS-PAGE. The composite immunoblots of Gc and Gn shown were overlaid as indicated. (B) TULV concentrated by ultracentrifugation was oxidized with 10 mM CuCl2 or thiol acetylated with NEM either with or without detergent TX-100 as indicated, and proteins were separated by SDS-PAGE without reductant as shown. Gc- and Gn-positive bands of ∼110 kDa and ∼200 kDa are indicated by asterisks. An enlargement of the region of >250 kDa from -SH (where SH is sulfhydryl) oxidized samples is shown as insert from the Gc immunoblot, highlighting novel bands positive for both Gn and Gc that appeared after CuCl2 treatment. (C) The virions after treatments, as in panel B, were reduced by the addition of 2 mM TCEP prior to SDS-PAGE. The composite immunoblots in panels B and C show Gc (left) overlaid with Gn (right). The images were recorded in an Odyssey infrared imaging system with goat anti-rabbit IR800CW-conjugated secondary antibody. The anti-Gc and anti-Gn serum reactivities were overlaid in PhotoShop CS using protein markers (Precision Plus Protein Standards; Bio-Rad) visible in both detections.