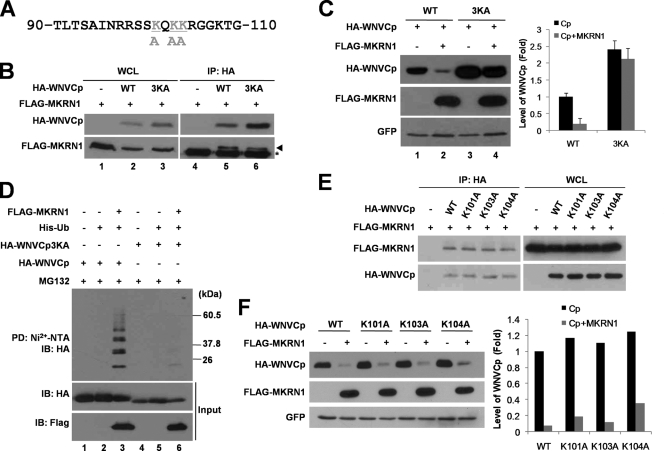
FIG. 7.
Lysines at residues 101, 103, and 104 are important for ubiquitination labeling and nuclear localization. (A) Sequence of WNVCp between positions 90 and 110. Gray shading indicates lysine residues replaced by alanine. (B) Interaction between 3KA and MKRN1. A plasmid expressing FLAG-MKRN1 was cotransfected with or without a plasmid expressing HA-WNVCp or HA-WNVCp 3KA in 293T cells. The whole-cell lysates (WCL) were immunoprecipitated (IP) with anti-HA antibodies. WCL and IP samples were detected with anti-HA and anti-FLAG antibodies. WT, wild type. (C) MKRN1-mediated degradation of 3KA. H1299 cells were transfected with a plasmid expressing FLAG-MKRN1 in the presence or absence of HA-WNVCp and 3KA. pEGFP-C2 expressing enhanced GFP was cotransfected as a transfection control. The cell lysates were detected with anti-HA, anti-FLAG, and anti-GFP antibodies. Protein quantification was performed as described for Fig. 1. Error bars indicate standard deviations. (D) Ubiquitination of WNVCp 3KA by MKRN1. Plasmids expressing HA-WNVCp, HA-WNVCp 3KA, FLAG-MKRN1, and His-Ub were transfected into H1299 cells. Cells were treated MG132 for 3 h before harvest. The cell lysates were pulled down (PD) with Ni2+-NTA resin. WCL and PD samples were detected with anti-HA and anti-FLAG antibodies. (E) Interaction between WNVCp point mutants and MKRN1. A plasmid expressing FLAG-MKRN1 was transfected with or without HA-WNVCp or its point mutants in 293T cells. WCL were immunoprecipitated with anti-HA antibodies. WCL and IP were detected with anti-HA and anti-MKRN1 antibodies. (F) Degradation of WNVCp point mutants by MKRN1. Mock vector or plasmids expressing FLAG-MKRN1, HA-WNVCp, or HA-WNVCp point mutants were transfected into H1299 cells. pEGFP-C2 expressing enhanced GFP was cotransfected as a transfection control. The lysates were detected with anti-HA antibodies, anti-FLAG antibodies, and anti-GFP antibodies.