Abstract
In immunosuppressed hosts, mucormycosis is a life-threatening infection with few treatment options. We studied the activity of colistin (polymyxin E) against Mucorales species in vitro and in a murine model of pulmonary Rhizopus oryzae infection. Colistin exhibited fungicidal activity in vitro against Mucorales spores and mycelia. At the colistin MIC, initial R. oryzae hyphal damage was followed by rapid regrowth; however, regrowth was prevented by combining colistin with a subinhibitory concentration of amphotericin B. Using electron microscopy and FM4-64 staining, we demonstrated that colistin disrupts R. oryzae cytoplasmic and vacuolar membranes, resulting in the leakage of intracellular contents. The prophylactic intranasal treatment of immunosuppressed mice with colistimethate significantly reduced the mortality rate and pulmonary fungal burden resulting from inhalational challenge with R. oryzae spores, whereas intraperitoneal colistimethate treatment had no effect. We conclude that colistin has modest in vitro and in vivo fungicidal activity against Mucorales spp. Further studies are warranted to assess the use of this drug in the prevention and treatment of mucormycosis.
During the past decade, pulmonary mucormycosis (PMM) has emerged as an important life-threatening opportunistic infection in severely immunocompromised patients, such as those with prolonged neutropenia and recipients of allogeneic hematopoietic stem cell transplantation (12, 21). In contrast to diabetic patients who develop rhinocerebral mucormycosis, surgical debridement is impractical in patients with PMM, and the correction of the underlying immunosuppression is challenging (12, 21); therefore, antifungal chemotherapy remains the chief therapeutic intervention for many patients with PMM. Unfortunately, Mucorales species are inherently resistant to most clinically available antifungal agents except amphotericin B, which is associated with renal and infusional toxicity, and posaconazole, which is available only as an oral formulation and has variable absorption from the gastrointestinal tract (12, 21). Hence, there is an urgent need for novel drugs with activity against Mucorales spp.
Polymyxins are cyclic, positively charged peptide antibiotics produced by the bacterium Bacillus polymyxa (16). Polymyxins bind to and disrupt the anionic outer cell membrane of Gram-negative bacteria and have a neutralizing effect on bacterial lipopolysaccharides (16). Interestingly, polymyxin B also has been shown to disrupt the cytoplasmic and vacuolar membranes of the model yeast Saccharomyces cerevisiae (23), suggesting that its antimicrobial activity extends to eukaryotic organisms. We studied the activity of polymyxin E (colistin) against Mucorales spp. in vitro and in a murine model of PMM.
MATERIALS AND METHODS
Isolates.
We performed susceptibility testing on 21 clinical Mucorales isolates recovered from patients at The University of Texas M. D. Anderson Cancer Center (Houston). Isolates were identified by standard morphological criteria, and the genus identification was confirmed by the sequencing of the ITS1-5.8S-ITS2 region of the fungal rRNA genes as described previously (13). The following isolates were tested: Rhizopus spp. (14 isolates), Mucor spp. (4 isolates), and Cunninghamella spp. (3 isolates). For comparison, four Aspergillus fumigatus and six A. terreus clinical isolates also were tested. Rhizopus oryzae 5799, a clinical strain that we previously used in a murine model of pulmonary infection (15), was selected for additional in vitro and animal studies, as detailed below. All isolates were grown on yeast extract agar glucose (YAG) plates for 48 to 72 h at 37°C. Spores were collected in sterile normal saline with 0.08% Tween 20, washed twice in normal saline, passed through 40-μm nylon filters (BD Biosciences, Franklin Lakes, NJ), and enumerated in a hemacytometer.
Drugs.
For in vitro experiments, stock solutions of colistin sulfate (15 mg/ml; Sigma, St. Louis, MO) and amphotericin B (5 mg/ml; Sigma) were prepared in sterile water, separated into aliquots, and stored at −20°C until use. Colistimethate, the inactive prodrug of colistin sulfate, is a less toxic formulation used for parenteral treatment (16). Therefore, for in vivo studies, colistimethate (X-gen Pharmaceuticals, Big Flats, NY) was reconstituted in sterile water at 30 mg/ml. Drugs were protected from light until use.
Susceptibility testing.
Broth microdilution was performed as outlined by the Clinical and Laboratory Standards Institute (4). Serial colistin dilutions and drug-free controls were prepared in RPMI 1640 with glucamine and without bicarbonate (64 to 0.06 μg/ml) or in yeast nitrogen base (YNB) complete medium (128 to 0.12 μg/ml) and dispensed in microtiter plates (100 μl per well). Freshly collected spores were suspended in the test medium at 0.5 × 104 to 4 × 104 cells/ml, and 100 μl was inoculated into each drug-containing well and drug-free control wells. Plates were incubated at 37°C for 24 h, and the MIC was determined visually as the lowest drug concentration resulting in 100% growth inhibition. The colistin minimum fungicidal concentration (MFC) was determined as described previously (5). Briefly, 20-μl suspensions from each well that showed the complete inhibition of growth and from the last positive well (showing growth similar to that in the control well) were subcultured onto YAG plates. The MFC was defined at 24 h as the lowest drug concentration at which fewer than three colonies were observed, which corresponded to a killing activity of ≥99.9%.
To study the interaction between colistin and amphotericin B against R. oryzae, we performed checkerboard experiments with microtiter plates containing combinations of colistin (0.06 to 64 μg/ml) and amphotericin B (0.125 to 8 μg/ml), as previously described (3). The fractional inhibitory concentration index (FICI) was calculated for checkerboard plates; values were interpreted as follows: FICI ≤ 0.5, synergy; 0.5 ≤ FICI < 4, indifference; and FICI > 4, antagonism (3, 18).
Although the poor agar diffusion characteristics of colistin limit the accuracy of disk diffusion assays, this method still may provide useful susceptibility data (7). Disk diffusion assays were performed using sterile paper disks (Fisher Scientific, Rochester, NY) containing 8 to 32 μg of colistin and RPMI 1640 plates inoculated with 200 μl of spore suspension (106 cells/ml). Inhibition zones were measured after 24 h of incubation at 37°C.
DiBAC viability staining.
To determine whether colistin exerts fungicidal activity against R. oryzae mycelia, we performed viability staining with bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC) (Molecular Probes, Carlsbad, CA) on drug-exposed and unexposed mycelia, as previously described (2). R. oryzae spores were grown to mycelia in microcentrifuge tubes with RPMI 1640 plus 0.15% (wt/vol) Junlon (Nihon Junyaku, Tokyo, Japan) at 37°C with shaking for 18 h. Tubes were centrifuged at 13,000 × g to remove the media, and mycelia were resuspended in RPMI containing colistin at the MIC or MFC; amphotericin B (2 μg/ml) and drug-free medium served as the positive and negative controls, respectively. Tubes were incubated for 6 h at 37°C with shaking. Mycelia next were washed twice in 3-(N-morpholino) propanesulfonic acid (MOPS) at pH 7 (designated MOPS7), and DiBAC was added from 1 mg/ml stock in 100% ethanol at 2 μg/ml in MOPS7. Tubes were incubated with gentle shaking (50 to 100 rpm) at ambient temperature in the dark for 1 h. Samples then were washed twice in MOPS7 and stored on ice until fluorescent microscopy. Images were acquired under a triple-band fluorescent microscope (Olympus BX-51; Olympus, Melville, NY) using the fluorescein isothiocyanate (FITC) filter.
Hyphal damage determined by XTT reduction assay.
We determined the time course of colistin-induced hyphal damage with the use of the 2,3-bis[2-methyloxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium-5-carboxanilide] (XTT) colorimetric formazan reduction assay. R. oryzae mycelia were suspended in RPMI 1640 containing various concentrations of colistin, amphotericin B, or both and incubated in microcentrifuge tubes at 37°C with shaking. Drug-free RPMI 1640 was used as the control medium. At the 0-, 2-, 6-, and 24-h time points, tubes were removed from incubation and centrifuged at 13,000 × g for 5 min. The medium was aspirated, and 1 mg of XTT (Sigma) plus 0.17 mg of menadione (Sigma) was added in 1 ml of phosphate-buffered saline (PBS) to each tube. Tubes were incubated at 37°C for an additional 1 h, and absorbance was measured at 405 nm. Hyphal viability for each time point and drug concentration was calculated as the optical density at 405 nm (OD405) of the drug-containing well divided by the OD405 of the control well. All experiments were performed at least three times.
The combined effects of colistin and amphotericin B were calculated as follows. For each experiment, the hyphal damage produced by each drug alone after 24 h of exposure was calculated and compared to the effect of treatment with the combination of both drugs. Synergism was defined as an antifungal effect of the combination treatment that was greater than the sum of the effects of each drug alone. An additive effect was defined as an antifungal effect of the combination treatment that was greater than the effect produced by either drug alone but that did not reach synergism. Antagonism was defined as an antifungal effect of the combination which was less than the effect produced by either drug alone (24).
Post-antifungal effect.
To determine the period of fungal growth inhibition after colistin exposure, we incubated R. oryzae spores (106/ml) in RPMI 1640 with colistin for 60 min, washed the spores three times in RPMI to remove the drug, and resuspended them in drug-free RPMI. The spore suspension was inoculated into 96-well plates (100 μl/well) and incubated at 37°C. The OD405 was measured every 10 min in a spectrophotometer (Power Wave HT; Biotek, Winooski, VT). Wells containing RPMI alone were used to correct for background absorbance. The lag time to the initiation of logarithmic growth was determined for each drug concentration, and the post-antifungal effect period was calculated as the lag time of the drug-containing well minus the lag time of the drug-free well.
ATP release assay.
We next measured intracellular ATP efflux from colistin-exposed germlings as an indication of cell membrane damage and cytoplasmic leakage (11). R. oryzae spores were enumerated in a hemacytometer and suspended in RPMI 1640 at 107 cells/ml. Spore suspensions then were incubated at 37°C and observed periodically until most cells had germinated (∼6 h). Germlings were separated from the medium by centrifugation and resuspended in 1-ml aliquots of RPMI 1640 containing colistin at the MIC or MFC and in drug-free RPMI at 37°C with shaking. The cells were removed by centrifugation after 5, 30, 60, and 90 min of incubation, and the supernatants were assayed for ATP using the CellTiter-Glo luminescent kit (Promega, Madison, WI). Data were collected with a microplate luminometer (Spectramax M5; Molecular Devices, Sunnyvale, CA).
Electron microscopy.
We observed the structural features of R. oryzae germlings grown in the presence of colistin and drug-free controls by transmission electron microscopy. Freshly collected R. oryzae spores were suspended in RPMI 1640 in microcentrifuge tubes at a final concentration of 105 cells/ml and incubated at 37°C for 6 h to generate germlings. The cells then were incubated with RPMI 1640 containing colistin at the MIC or MFC for an additional 60 min at 37°C with shaking. Tubes next were centrifuged at 13,000 × g for 5 min to remove the medium, and the pellet was washed twice in MOPS7. Samples were fixed with a solution containing 3% glutaraldehyde plus 2% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.3. After fixation, the samples were washed in 0.1 M cacodylate buffer, postfixed in 4% Millipore-filtered potassium permanganate for 1 h, washed in distilled water, and stained en bloc with 1% Millipore-filtered aqueous uranyl acetate for 1 h. The samples were dehydrated in increasing concentrations of ethanol, changed three times in propylene oxide, infiltrated, and embedded in LX-112 medium. Samples were polymerized in a 70°C oven for 3 days. Ultrathin sections were cut in a Leica Ultracut microtome (Leica, Deerfield, IL), stained with uranyl acetate and lead citrate in a Leica EM stainer, and examined with a JEM 1010 transmission electron microscope (JEOL, USA, Inc., Peabody, MA) at an accelerating voltage of 80 kV. Digital images were obtained using the AMT Imaging System (Advantage Microscopy Techniques Corp., Danvers, MA).
FM4-64 vacuolar staining.
We next used the lipophilic styryl dye N-(3-triethylammoniumpropyl)-4-(p-diethylaminophenylhexatrienyl)pyridinium dibromide (FM4-64) to visualize vacuolar changes in colistin-exposed R. oryzae germlings (25). A stock solution of FM4-64 (Molecular Probes) was prepared in MOPS at 20 μg/ml. R. oryzae germlings were generated and exposed to colistin, as detailed above. Germlings were suspended in RPMI 1640 with 5 μM FM4-64 at a final concentration of 5 × 106/ml and incubated for 30 min at 30°C in the dark with shaking. Germlings were centrifuged at 13,000 × g for 5 min, washed in PBS to remove excess dye, resuspended in RPMI 1640 with colistin (at the MIC and MFC) or drug-free RPMI, and incubated for 60 min at 37°C with shaking. Finally, cell suspensions were mounted on glass slides and observed at various magnifications under a triple-band fluorescent microscope (Olympus) using the rhodamine filter.
Murine model of PMM.
Finally, we studied the efficacy of colistin in a murine model of PMM (17). Female BALB/c mice weighing 18 to 22 g were immunosuppressed with 75 mg/kg of body weight cyclophosphamide intraperitoneally (i.p.) 4 and 1 day prior to inoculation and 300 mg/kg of cortisone acetate subcutaneously 1 day prior to inoculation. Mice were anesthetized with 2% isoflurane inhalation and inoculated intranasally with a 35-μl droplet containing ∼3,500 R. oryzae spores. Additional cyclophosphamide (75 mg/kg, i.p.) was administered 2 and 5 days after inoculation.
To determine dosing regimens for colistimethate in mice, we measured plasma colistin levels in 24 mice after the bolus i.p. injection of colistimethate at 80 mg/kg. Mice were sacrificed and blood was drawn by cardiac puncture 2, 4, 6, and 24 h after colistimethate injection. The plasma was separated by centrifugation, and tubes were stored at −80°C until the assay was performed. The colistimethate concentration was determined using high-performance liquid chromatography (HPLC). This method involves pretreatment with trichloroacetic acid in methanol-water (50:50, vol/vol) and the accelerated hydrolysis of colistimethate to colistin, followed by the determination of colistin A and B concentrations using the Alliance HPLC system (e2695 separations module and EMD 1000 mass spectrometer; Waters, Milford, MA). The system was calibrated using mouse plasma spiked with colistimethate at 0.25, 0.5, 1.0, 2.5, 5.0, 7.5, 10.0, 12.5, and 15 μg/ml. This analysis revealed the rapid clearance of colistimethate in mice, with a plasma half life of only 11 min and an area under the curve of 192 μg/ml per min. While these results suggested a need for frequent colistimethate dosing, we encountered a high frequency of neurotoxicity when colistimethate 30 mg/kg was given every 8 h. Therefore, we chose a colistimethate dose of 40 mg/kg every 12 h for systemic treatment experiments, as recently reported by another group (1).
Two colistimethate regimens designed to simulate treatment and prophylaxis strategies were evaluated. The treatment and placebo groups included 10 mice each; experiments were repeated three times. The treatment arm consisted of 40 mg/kg colistimethate administered i.p. every 12 h for 5 days starting 3 h after R. oryzae inoculation. Control mice received i.p. injections of sterile normal saline at time points similar to those used for the treatment group. The prophylaxis arm consisted of colistimethate given intranasally at 2.5 mg/kg every 12 h (1) for 5 days starting 1 day prior to inoculation; on the day of inoculation, colistin was instilled intranasally 3 h after R. oryzae spores. Mortality was monitored twice daily for 10 days. Moribund mice were euthanized by CO2-induced asphyxiation, and their deaths were recorded as occurring 12 h later. All procedures were performed according to the highest standards for humane handling, care, and treatment of research animals and were approved by the M. D. Anderson Cancer Center institutional animal care and use committee.
Mouse lungs were excised, and the fungal burden was measured using real-time quantitative PCR (RT-qPCR) for the R. oryzae rRNA gene, as previously described (15). In brief, DNA was extracted from lung homogenates using the DNeasy tissue kit (Qiagen, Valencia, CA), and DNA samples were analyzed in duplicate using the ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA). Primers and dual-labeled fluorescent hybridization probes specific for R. oryzae 18S rRNA were designed using the ABI PRISM SeqScape software program (version 2; Applied Biosystems). The oligonucleotide sequences were the following: (i) sense amplification primer, 5′-AGGGCTTTGCTGCACGAC-3′; (ii) antisense amplification primer, 5′-TCCACCTCGGGACTGTTTG-3′; and (iii) hybridization probe, 5′-6-carboxyfluorescein-TGCTACTGTTGCAGACCGCCGCT-6-carboxytetramethylrhodamine-3′. The threshold cycle (CT) of each sample was interpolated from a 6-point standard curve of CT values prepared by spiking uninfected mouse lungs with 102 to 107 R. oryzae spores. The RT-qPCR results were reported as spore equivalents of R. oryzae DNA.
Statistical analysis.
Continuous variables with normal value distribution (hyphal viability and ATP concentration in supernatants) were compared between treatment and control groups using Student's t test. Pulmonary fungal burdens, which had a nonnormal value distribution, were compared among the different animal groups using the nonparametric Kruskal-Wallis test, and Dunn's test was used for post hoc comparisons between each treatment group and the control group. Calculations were performed using InStat (GraphPad, San Diego, CA). Survival curves were plotted using the Kaplan-Meier method. The survival curves of different treatment groups were compared using the log-rank test. Two-sided P values of <0.05 were considered statistically significant.
RESULTS
Colistin has antifungal activity in vitro against Mucorales spores and mycelia.
Susceptibility testing by broth microdilution in RPMI 1640 generally revealed clear end points with complete growth inhibition. For Mucorales isolates, the median colistin MIC was 16 μg/ml (range, 8 to 32 μg/ml) (Table 1). Colistin was fungicidal at concentrations of one to four times the MIC (median MFC/MIC ratio, 2). In contrast, the colistin MICs for all Aspergillus isolates tested were ≥64 μg/ml (data not shown). Colistin MICs against all Mucorales isolates were higher (128 μg/ml) when YNB was used as the test medium (Table 1). Disk diffusion testing yielded clear zones of inhibition with a median diameter of 18 mm for 8-μg colistin disks, 19 mm for 16-μg colistin disks, and 21 mm for 32-μg colistin disks (Fig. 1).
TABLE 1.
Colistin MICs and MFCs for 21 clinical Mucorales isolates
| Isolate | Test mediuma |
|||
|---|---|---|---|---|
| RPMI 1640 |
YNB |
|||
| MIC | MFC | MIC | MFC | |
| Cunninghamella bertholletiae 5633 | 16 | 64 | 128 | >128 |
| Cunninghamella bertholletiae 6694 | 16 | 64 | 128 | >128 |
| Cunninghamella bertholletiae 6733 | 32 | 64 | 128 | >128 |
| Mucor circinelloides 4030 | 16 | 64 | 128 | >128 |
| Mucor circinelloides 4480 | 16 | 32 | 128 | >128 |
| Mucor circinelloides 4818 | 32 | 32 | 128 | >128 |
| Mucor circinelloides 5904 | 16 | 64 | 128 | >128 |
| Rhizopus oryzae 4549 | 32 | 64 | 128 | >128 |
| Rhizopus oryzae 3140 | 16 | 32 | 128 | >128 |
| Rhizopus oryzae 4153 | 16 | 64 | 128 | >128 |
| Rhizopus sp. 4523 | 16 | 64 | 128 | >128 |
| Rhizopus oryzae 5879 | 32 | 32 | 128 | >128 |
| Rhizopus homothallicus 5790 | 16 | 64 | 128 | >128 |
| Rhizopus homothallicus 5910 | 32 | 32 | 128 | >128 |
| Rhizopus oryzae 5799 | 8 | 32 | 128 | >128 |
| Rhizopus oryzae 5496 | 16 | 32 | 128 | >128 |
| Rhizopus microsporus 6164 | 32 | 64 | 128 | >128 |
| Rhizopus oryzae 6093 | 16 | 32 | 128 | >128 |
| Rhizopus oryzae 6725 | 16 | 32 | 128 | >128 |
| Rhizopus homothallicus 6360 | 32 | 64 | 128 | >128 |
| Rhizopus bertholletiae 6254 | 16 | 32 | 128 | >128 |
MICs and MFCs are in micrograms/milliliter.
FIG. 1.
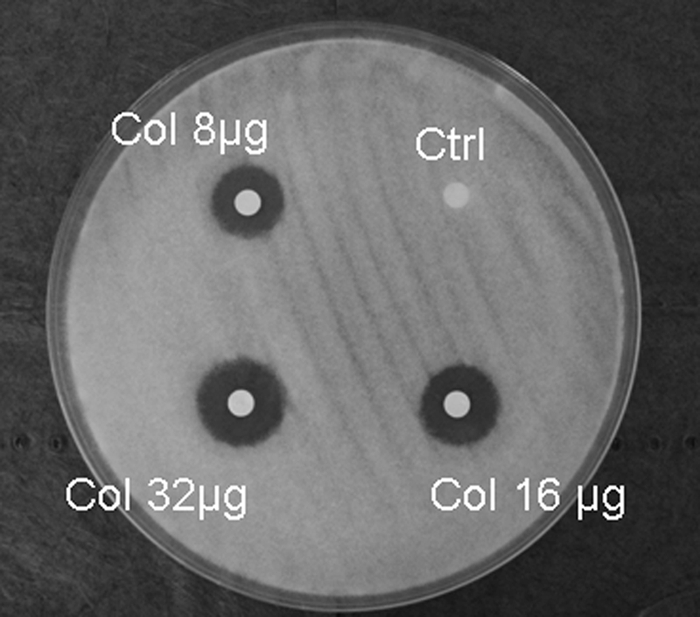
Colistin disk diffusion assay. Paper disks containing 8, 16, and 32 μg of colistin (Col) were placed on yeast extract agar glucose plates inoculated with a 106/ml R. oryzae spore suspension. Clear inhibition zones, measured 24 h later, revealed a colistin dose-response relationship.
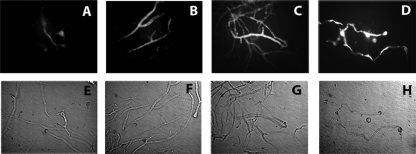
DiBAC vital staining revealed enhanced uptake in colistin-exposed R. oryzae hyphae compared to that in non-colistin-exposed hyphae. Extensive DiBAC uptake, indicating fungicidal drug activity, was observed at a colistin concentration of 32 μg/ml; i.e., the MFC for the R. oryzae 5799 strain (Fig. 2).
FIG. 2.
DiBAC viability stain. R. oryzae mycelia were exposed to colistin at the MIC (8 μg/ml) (B) or MFC (32 μg/ml) (C) for 6 h; amphotericin B (2 μg/ml) (D) and non-drug-exposed mycelia (A) served as controls. Mycelia subsequently were stained with DiBAC and observed with fluorescent (A to D) and bright-field microscopy (E to H) (×400 magnification). Colistin-exposed mycelia were enhanced with DiBAC in a dose-dependent manner, which is consistent with fungicidal drug activity. The fungicidal activity of 32 μg/ml colistin was similar to that of 2 μg/ml amphotericin B.
R. oryzae growth inhibition by colistin is followed by rapid regrowth.
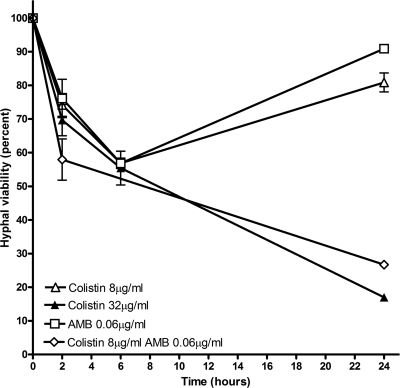
We observed no delay in the onset of the logarithmic growth phase for R. oryzae spores after colistin exposure relative to that of non-colistin-exposed R. oryzae spores; this finding is consistent with the absence of a post-antifungal effect for colistin. XTT reduction assays demonstrated a reduction of ∼45% in hyphal viability during the initial 6 h of exposure to 8 μg/ml colistin; however, this initial reduction in viability was followed by rapid regrowth at 24 h (Fig. 3). Exposure to colistin at 32 μg/ml (the MFC) resulted in a more sustained reduction in hyphal viability of ∼80% at 24 h (Fig. 3).
FIG. 3.
XTT reduction assay. XTT reduction assays were performed on R. oryzae mycelia exposed to various colistin and amphotericin B concentrations. Hyphal damage was determined at each time point as detailed in Materials and Methods. Exposure to 8 μg/ml of both colistin (open triangles) and amphotericin B (open squares) resulted in an initial reduction in hyphal viability, followed by increased viability at 24 h. Exposure to 32 μg/ml colistin (solid triangles) resulted in a more sustained reduction in hyphal viability at 24 h. The combination of 8 μg/ml colistin and 0.06 μg/ml amphotericin B (open diamonds) induced a sustained reduction in hyphal viability similar to that induced by 32 μg/ml colistin alone. AMB, amphotericin B.
The combination of amphotericin B (0.06 μg/ml) and colistin (8 μg/ml) had synergistic activity against R. oryzae mycelia, resulting in sustained reduction in hyphal viability at 24 h that was similar to that achieved with 32 μg/ml colistin (Fig. 3). Concordant results were obtained using the checkerboard methodology; the combination of colistin and amphotericin B produced FICI values of 0.28 to 0.31, which is consistent with the synergistic activity of this drug combination against R. oryzae spores in vitro.
Colistin damages R. oryzae cytoplasmic and vacuolar membranes.
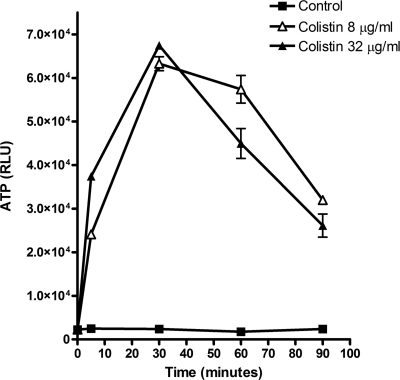
The rapid efflux of ATP was detected 5 min after the incubation of R. oryzae germlings with colistin. The ATP concentration in culture supernatants peaked after 30 min of coincubation with colistin and declined thereafter (Fig. 4). These results are consistent with a rapid increase in the plasma membrane permeability of germlings coincubated with colistin.
FIG. 4.
ATP release assay. The incubation of R. oryzae germlings with colistin at 8 (open triangles) or 32 μg/ml (solid triangles) resulted in rapid ATP release from the cells, indicating cell membrane disruption and plasma leakage. ATP was measured in the suspension with the CellTiter-Glo luminescent assay (Promega). RLU, relative light units.
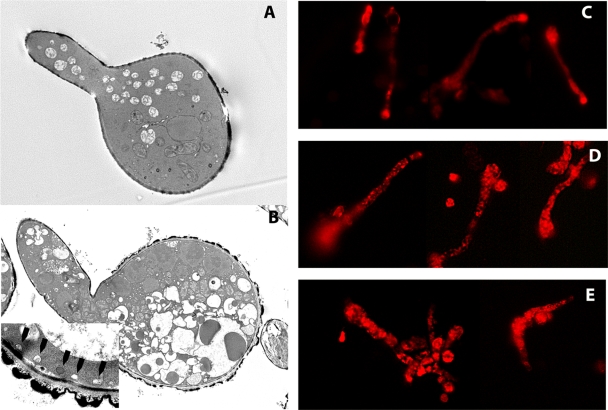
We next studied the morphological changes in colistin-exposed R. oryzae germlings using transmission electron microscopy. Compared to those of non-drug-exposed germlings, a marked increase in the number and size of vacuoles was seen in colistin-treated germlings (Fig. 5). Moreover, the vacuoles of colistin-treated germlings appeared ruffled and dysmorphic. These changes were most severe in germlings exposed to colistin at the MFC, in which much of the cytoplasm was occupied by swollen vacuoles (Fig. 5). In addition, we observed bleb formation adjacent to the cell membrane, suggesting cell membrane disruption in colistin-exposed R. oryzae germlings. To confirm these observations, we performed fluorescent microscopy with FM4-64, which stains cellular and vacuolar membranes (25). In non-colistin-exposed germlings, FM4-64 was concentrated in the plasma membrane and germ tube tips, reflecting the known aggregation of secretory vesicles in Mucorales hyphal tips (9). In contrast, in colistin-exposed germlings, FM4-64 was redistributed to vacuoles throughout the cytoplasm, whereas the cell membrane and hyphal tips were not enhanced by the dye (Fig. 5). At the highest colistin concentrations, vesicles appeared swollen and malformed, and FM4-64 was dispersed in the cytoplasm, indicating vacuolar damage and disruption (Fig. 5).
FIG. 5.
Colistin induces vacuolar and plasma membrane damage. Transmission electron microscopy was performed on R. oryzae germlings after exposure to various colistin concentrations. (A) Non-drug-exposed germling (×5,000 magnification). (B) Colistin induced extensive cytoplasmic vacuolar swelling (×5,000 magnification) and bleb formation near the plasma membrane (arrowheads; inset, ×20,000 magnification). Fluorescent staining with the styryl dye FM4-64 enhanced the visualization of vacuoles. (C) In untreated germlings, FM4-64 concentrated in the plasma membrane and germ tube tips. (D) After being exposed to 8 μg/ml colistin, FM4-64 shifted to multiple cytoplasmic vacuoles. (E) Exposure to a higher colistin concentration (32 μg/ml) resulted in vacuolar disruption and the dispersal of the dye in the cytoplasm (×400 magnification).
Intranasal colistimethate protects mice from pulmonary R. oryzae infection.
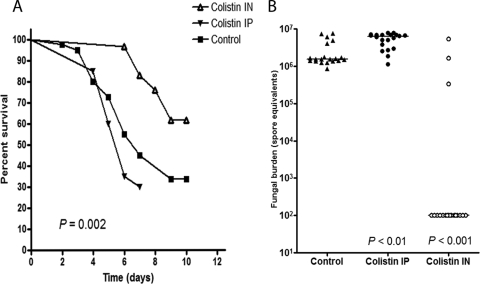
To determine whether the observed in vitro activity of colistin translates into meaningful activity in an animal mucormycosis model, we studied two colistimethate regimens in immunosuppressed mice. Treatment with i.p. colistimethate did not significantly affect the mortality rate of BALB/c mice infected intranasally with R. oryzae spores (Fig. 6A). In contrast, the prophylactic intranasal administration of colistimethate increased the survival rate at 10 days from 33 to 61% (P = 0.002 by the log-rank test) (Fig. 6A). Moreover, the pulmonary fungal burden of mice treated prophylactically with intranasal colistimethate was significantly lower than that of untreated mice (P < 0.001) and was below the assay's lower detection limit in 84% of mice in this treatment group. The fungal burden was increased in the lungs of mice treated with i.p. colistimethate relative to that of the control group (P < 0.01) (Fig. 6B).
FIG. 6.
In vivo activity of colistin in a murine model of pulmonary mucormycosis. The in vivo activity of colistin was studied in BALB/c mice immunosuppressed with cyclophosphamide and cortisone acetate and inoculated intranasally with R. oryzae spores. Treatment with intraperitoneal colistimethate (80 mg/kg/day) had no effect on the mortality rate in this model (A), whereas prophylaxis with intranasal colistimethate (5 mg/kg/day) significantly reduced the mortality rate (P = 0.002 by the log-rank test) (A) and pulmonary fungal burden, as determined by RT-qPCR (P < 0.001) (B). The pulmonary fungal burden was increased in mice treated with colistin i.p. relative to that of untreated control mice (P < 0.01).
DISCUSSION
We found that colistin has fungicidal in vitro activity against Mucorales spores and mycelia. The antifungal effect of colistin apparently was mediated by its disruptive activity on Mucorales cytoplasmic and vacuolar membranes. Moreover, the intranasal administration of colistimethate significantly reduced the mortality rate of immunosuppressed BALB/c mice challenged with R. oryzae spores. Taken together, these observations suggest that colistin has clinical applications in the prevention and treatment of mucormycosis in immunosuppressed hosts.
Mucorales isolates had colistin MICs of 8 to 32 μg/ml (MIC50, 16 μg/ml) in RPMI 1640; MFCs were one to four times the MICs (median MFC/MIC, 2). In human studies, single doses of 75 to 150 mg colistin base produced serum concentrations of bioactive colistin of 6 to 18 μg/ml (8, 20); higher colistin concentrations (13 to 32 μg/ml) were measured during The prolonged therapy of patients with cystic fibrosis (22). Thus, fungicidal concentrations of colistin may be difficult to achieve with intravenous dosing but may be attained locally in the lungs using aerosolized drug delivery.
Colistin MICs against Mucorales were affected by the test medium; MICs were significantly higher in YNB complete medium than those in RPMI 1640. The in vitro activity of colistin against Gram-negative bacteria is known to be affected by the concentrations of divalent cations, which compete for binding sites on the bacterial outer membrane (10). YNB contains higher concentrations of calcium and magnesium (0.9 and 4.1 mM, respectively) than those of RPMI 1640 (0.42 and 0.40 mM, respectively), which could explain the attenuated activity of colistin in YNB.
We found no post-antifungal effect of colistin against R. oryzae. Furthermore, XTT reduction assays revealed that the initial damage caused to R. oryzae hyphae as a result of exposure to colistin at the MIC was followed rapidly by fungal regrowth. This phenomenon was negated by the addition of a subinhibitory concentration of amphotericin B, which is consistent with the synergistic activity of these two drugs against R. oryzae. Interestingly, similar observations regarding the antibacterial activity of colistin (14, 19) have led researchers to suggest that colistin should be combined with other antimicrobial agents (14). Intranasal colistimethate prophylaxis in immunosuppressed mice reduced but did not completely prevent mortality from pulmonary mucormycosis, suggesting that this drug has modest antifungal activity in vivo. Therefore, colistin should be explored as an adjunct treatment for mucormycosis.
Colistin had no effect on mortality rates when administered i.p. after the intranasal inoculation of R. oryzae spores. This finding likely reflects the rapid clearance of systemically administered colistimethate observed in our pharmacokinetic experiment. The half-life of colistimethate in the lung after intranasal administration previously was shown to be significantly longer than that of systemically dosed colistimethate (50 versus 11 min) (1), which may explain the relative efficacy of the intranasal route in our animal model. In addition, the earlier (prophylactic) administration of intranasal colistimethate may have accounted for its efficacy.
The clinical use of colistin has increased in recent years because of its activity against multidrug-resistant Gram-negative bacteria (16). Newly manufactured colistin formulations are associated with lower rates of nephrotoxicity than historically reported rates (16), expanding the application of this drug to a wider range of critically ill patients. Colistin has been aerosolized for inhalational use in the treatment and prevention of pneumonia caused by Gram-negative bacteria (6). This mode of colistin administration may be particularly appealing for the adjunct treatment of sinopulmonary mucormycosis, because high drug concentrations are achieved in the airways with minimal systemic toxicity.
Unexpectedly, colistin had no in vitro activity against Aspergillus isolates. This selective activity suggests that the disruption of fungal membranes by colistin depends on the presence of specific receptors. Similarly, the activity of colistin against Gram-negative bacteria is facilitated by binding to the lipid A moiety of lipopolysaccharide; variations in lipid A structure render certain Gram-negative bacteria, such as Proteus mirabilis and Burkholderia cepacia, intrinsically resistant to colistin (16).
In conclusion, given the currently limited treatment options for mucormycosis in immunosuppressed patients, our findings should prompt further preclinical and clinical studies to evaluate the use of colistin for the treatment and prevention of this life-threatening infection.
Acknowledgments
We thank Kenneth Dunner, Jr., for his expert help with our electron microscopy studies.
This work was supported by Institutional Core Grant CA16672 from the High-Resolution Electron Microscopy Facility, M. D. Anderson Cancer Center.
Footnotes
Published ahead of print on 26 October 2009.
REFERENCES
- 1.Aoki, N., K. Tateda, Y. Kikuchi, S. Kimura, C. Miyazaki, Y. Ishii, Y. Tanabe, F. Gejyo, and K. Yamaguchi. 2009. Efficacy of colistin combination therapy in a mouse model of pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. J. Antimicrob. Chemother. 63:534-542. [DOI] [PubMed] [Google Scholar]
- 2.Bowman, J. C., P. S. Hicks, M. B. Kurtz, H. Rosen, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2002. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob. Agents Chemother. 46:3001-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamilos, G., R. E. Lewis, and D. P. Kontoyiannis. 2006. Lovastatin has significant activity against zygomycetes and interacts synergistically with voriconazole. Antimicrob. Agents Chemother. 50:96-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard, 2nd ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Espinel-Ingroff, A., A. Fothergill, J. Peter, M. G. Rinaldi, and T. J. Walsh. 2002. Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS collaborative study. J. Clin. Microbiol. 40:3204-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falagas, M. E., S. K. Kasiakou, S. Tsiodras, and A. Michalopoulos. 2006. The use of intravenous and aerosolized polymyxins for the treatment of infections in critically ill patients: a review of the recent literature. Clin. Med. Res. 4:138-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galani, I., F. Kontopidou, M. Souli, P. D. Rekatsina, E. Koratzanis, J. Deliolanis, and H. Giamarellou. 2008. Colistin susceptibility testing by Etest and disk diffusion methods. Int. J. Antimicrob. Agents 31:434-439. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin, N. J., and E. A. Friedman. 1968. The effects of renal impairment, peritoneal dialysis, and hemodialysis on serum sodium colistimethate levels. Ann. Intern. Med. 68:984-994. [DOI] [PubMed] [Google Scholar]
- 9.Grove, S. N., and C. E. Bracker. 1970. Protoplasmic organization of hyphal tips among fungi: vesicles and Spitzenkorper. J. Bacteriol. 104:989-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock, R. E., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins, D. L., R. Chang, D. V. Debabov, J. Leung, T. Wu, K. M. Krause, E. Sandvik, J. M. Hubbard, K. Kaniga, D. E. Schmidt, Jr., Q. Gao, R. T. Cass, D. E. Karr, B. M. Benton, and P. P. Humphrey. 2005. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kontoyiannis, D. P., and R. E. Lewis. 2006. Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management. Infect. Dis. Clin. North Am. 20:581-607. [DOI] [PubMed] [Google Scholar]
- 13.Kontoyiannis, D. P., M. S. Lionakis, R. E. Lewis, G. Chamilos, M. Healy, C. Perego, A. Safdar, H. Kantarjian, R. Champlin, T. J. Walsh, and I. I. Raad. 2005. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J. Infect. Dis. 191:1350-1360. [DOI] [PubMed] [Google Scholar]
- 14.Kroeger, L. A., L. B. Hovde, I. F. Mitropoulos, J. Schafer, and J. C. Rotschafer. 2007. Colistin methanesulfonate against multidrug-resistant Acinetobacter baumannii in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 51:3431-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamaris, G. A., R. Ben-Ami, R. E. Lewis, G. Chamilos, G. Samonis, and D. P. Kontoyiannis. 2009. Increased virulence of zygomycetes organisms following exposure to voriconazole: a study involving fly and murine models of zygomycosis. J. Infect. Dis. 199:1399-1406. [DOI] [PubMed] [Google Scholar]
- 16.Landman, D., C. Georgescu, D. A. Martin, and J. Quale. 2008. Polymyxins revisited. Clin. Microbiol. Rev. 21:449-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leventakos, K., R. E. Lewis, and D. P. Kontoyiannis. 2009. Effect of low and high dose of caspofungin (CAS) in a neutropenic and a steroid treated murine model of pulmonary mucormycosis (MCM). Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1690.
- 18.Lewis, R. E., D. J. Diekema, S. A. Messer, M. A. Pfaller, and M. E. Klepser. 2002. Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J. Antimicrob. Chemother. 49:345-351. [DOI] [PubMed] [Google Scholar]
- 19.Li, J., C. R. Rayner, R. L. Nation, R. J. Owen, D. Spelman, K. E. Tan, and L. Liolios. 2006. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 50:2946-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackay, D. N., and D. Kaye. 1964. Serum concentrations of colistin in patients with normal and impaired renal function. N. Engl. J. Med. 270:394-397. [DOI] [PubMed] [Google Scholar]
- 21.Pyrgos, V., S. Shoham, and T. J. Walsh. 2008. Pulmonary zygomycosis. Semin. Respir. Crit. Care Med. 29:111-120. [DOI] [PubMed] [Google Scholar]
- 22.Reed, M. D., R. C. Stern, M. A. O'Riordan, and J. L. Blumer. 2001. The pharmacokinetics of colistin in patients with cystic fibrosis. J. Clin. Pharmacol. 41:645-654. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz, S. N., G. Medoff, G. S. Kobayashi, C. N. Kwan, and D. Schlessinger. 1972. Antifungal properties of polymyxin B and its potentiation of tetracycline as an antifungal agent. Antimicrob. Agents Chemother. 2:36-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simitsopoulou, M., E. Roilides, A. Maloukou, C. Gil-Lamaignere, and T. J. Walsh. 2008. Interaction of amphotericin B lipid formulations and triazoles with human polymorphonuclear leucocytes for antifungal activity against Zygomycetes. Mycoses 51:147-154. [DOI] [PubMed] [Google Scholar]
- 25.Vida, T. A., and S. D. Emr. 1995. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128:779-792. [DOI] [PMC free article] [PubMed] [Google Scholar]