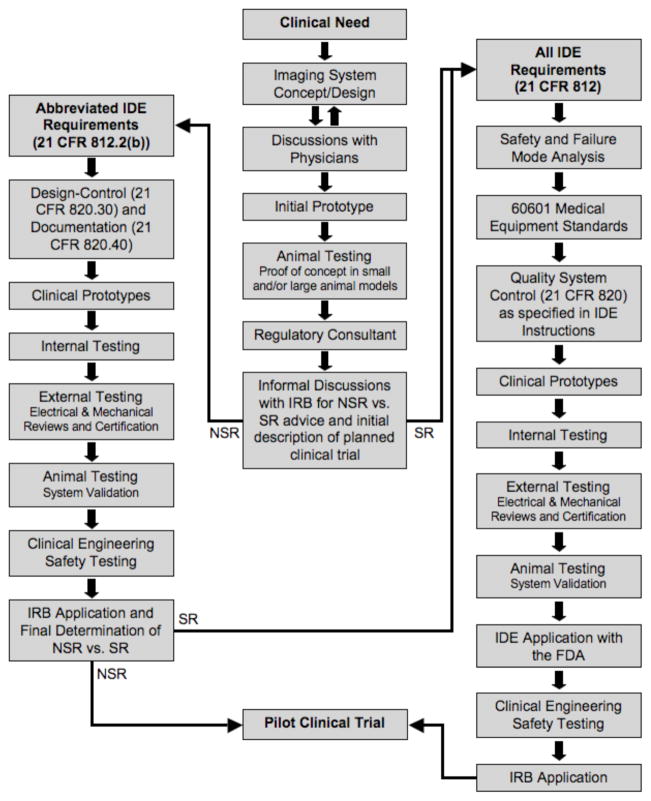
Fig. 1.
Flow chart for first-in-human testing of a new device for medical imaging, starting with clinical need and ending with a pilot clinical trial. SR vs. NSR status is a key decision point early in the process, but a binding determination by the IRB can only occur after a full application is filed (the “risk conundrum”).