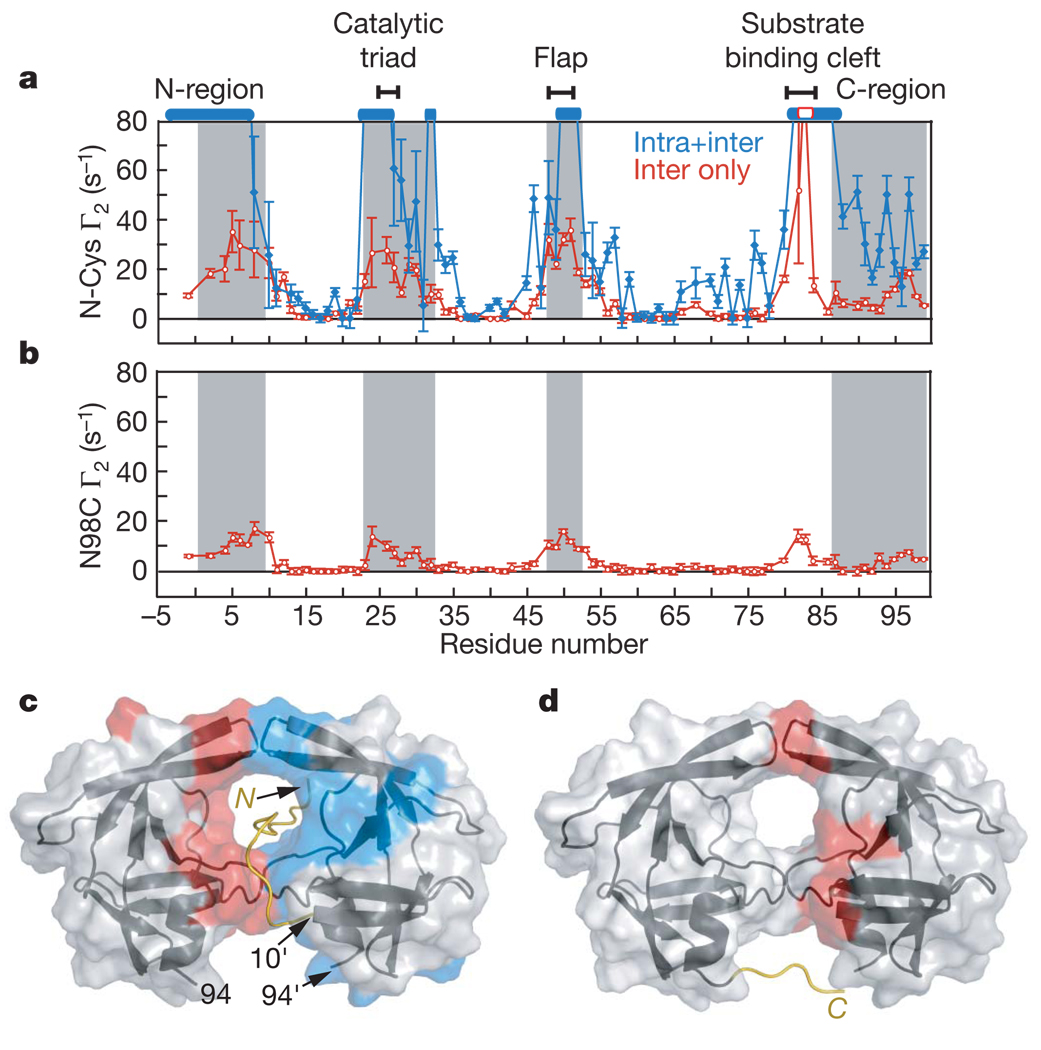
Figure 3. PRE profiles with spin labels attached at the N- and C termini of the SFNFPR(D25N) mini-precursor.
a, Intermolecular PREs (red) observed for a 1:1 mixture (0.2 mM each) of N-terminal spin-labelled S(C)FNFPR(D25N) at natural isotopic abundance and U-[2H/13C/15N]–SFNFPR(D25N), and the sum of the inter- and intramolecular PREs (blue) observed for 0.2 mM N-terminal spin-labelled U-[2H/13C/15N]–S(C)FNFPR(D25N). Residues broadened beyond detection are denoted by open bars. b, Intermolecular PREs observed for a 1:1 mixture (0.2 mM each) of U-[2H/13C/15N]–SFNFPR(D25N) and C-terminal spin-labelled (at N98C) SFNFPR(D25N) at natural isotopic abundance. Grey shaded areas in a and b delineate residues that are buried at the dimer interface in the mature protease. Error bars in a and b represent 1 s.d. c, d, Inter- and intramolecular PREs with Γ2 rates >10 s−1 colour-coded in red and blue, respectively, onto the molecular surface of the mature protease dimer originating from the N-terminal (c) and the C-terminal (d) spin labels. The intramolecular PRE rates are given by the difference in PRE rates between the blue and red profiles in a. Cartoons of modelled N-terminal (residues −4 to 9) and C-terminal (residues 95–99) regions bearing the spin labels are included in c and d, respectively.