Abstract
Beauveria bassiana is an important entomopathogenic fungus widely used as a biological agent to control insect pests. A gene (B. bassiana JEN1 [BbJEN1]) homologous to JEN1 encoding a carboxylate transporter in Saccharomyces cerevisiae was identified in a B. bassiana transfer DNA (T-DNA) insertional mutant. Disruption of the gene decreased the carboxylate contents in hyphae, while increasing the conidial yield. However, overexpression of this transporter resulted in significant increases in carboxylates and decreased the conidial yield. BbJEN1 was strongly induced by insect cuticles and highly expressed in the hyphae penetrating insect cuticles not in hyphal bodies, suggesting that this gene is involved in the early stage of pathogenesis of B. bassiana. The bioassay results indicated that disruption of BbJEN1 significantly reduced the virulence of B. bassiana to aphids. Compared to the wild type, ΔBbJEN1 alkalinized the insect cuticle to a reduced extent. The alkalinization of the cuticle is a physiological signal triggering the production of pathogenicity. Therefore, we identified a new factor influencing virulence, which is responsible for the alkalinization of the insect cuticle and the initiation of fungal pathogenesis in insects.
Mycoinsecticides are considered promising biological control agents and alternatives or supplements to chemical pesticides (15). However, the dearth of physiological, genetic, and molecular knowledge of entomopathogenic fungi has retarded their widespread application.
For mycoinsecticide improvement, greater attention and effort have been given to elucidate the mechanisms of fungal pathogenesis (13, 14, 18, 20, 29, 49, 50, 51, 52, 53). Entomopathogenic fungi, e.g., Metarhizium anisopliae and Beauveria bassiana, invade their hosts by direct penetration of the host exoskeleton or cuticle. M. anisopliae and B. bassiana produce hydrophobic spores which contact and adhere to the insect cuticle (12). Once attached, the conidium germinates and the germ tubes differentiate into swollen infection structures called appressoria. The appressoria produce penetration pegs which penetrate the insect cuticle via cuticle-degrading enzymes (11, 19, 46) as well as mechanical pressure (24, 53). Hyphae proliferate within the hemocoel, emerge from inside the insect, and subsequently conidiate on the cadaver (15). However, much remains to be elucidated regarding the mechanisms of insect fungal pathogenesis.
To obtain detailed knowledge of the mechanisms of fungal pathogenesis, a pool of B. bassiana transfer DNA (T-DNA) insertional mutants had been generated through an Agrobacterium-mediated-transformation method (21). A mutant, designated T12, characterized by the presence of more conidia, was isolated, and its flanking sequence was obtained by T-DNA tagging. The flanking fragment contained an open reading frame (ORF), which corresponded to a gene termed JEN1, encoding a transporter of carboxylates (http://www.ncbi.nlm.nih.gov/Blast.cgi). Organic acid transportation is important for the metabolism of almost all cells of multicellular organisms and unicellular microorganisms (17, 25, 26). Transport across the plasma membrane is the first step in the metabolism of these substrates, which may affect many aspects of the organism, including regulation of energy metabolism (9, 34) and acid-base equilibrium status (10).
JEN1p has been identified in several fungal species, e.g., Saccharomyces cerevisiae, Candida albicans, and Kluyveromyces lactis (9, 35, 45), which is a lactate/pyruvate symporter (1, 9, 34). The enzyme imports lactate or some short-chain monocarboxylates across the plasma membrane into cells. Then, the lactate is stereo-specifically oxidized to pyruvate. This reaction is performed by ferricytochrome c oxidoreductase in mitochondria (23, 33) and is tightly connected to the respiratory chain (34). JEN1 was induced by lactic, pyruvic, acetic, and propionic acids and repressed by glucose (2, 9, 35, 45). Nevertheless, for entomopathogenic fungi, the characterization of JEN1p has not been investigated, and its role in infection is still a mystery.
For this paper, we studied the functions of a putative carboxylate transport gene, JEN1, in B. bassiana (BbJEN1). Our results demonstrated that BbJEN1 is involved in conidiation of B. bassiana and that the gene is a new factor influencing virulence in entomopathogenic fungi.
MATERIALS AND METHODS
Strains, growth conditions, and DNA manipulations.
B. bassiana wild-type strain Bb0062 and its cultivation were previously described (19). For the repression and derepression study, conidia were cultivated in liquid Sabouraud's dextrose supplemented with 1% (wt/vol) yeast extract (SDY medium; pH 7.0) at 26°C on a rotary shaker at 180 rpm for about 48 h, and the hyphae were harvested (0.2 ± 0.01 g [wet weight]) and then transferred to 25-ml volumes of minimal medium (0.5% sodium nitrate, 0.03% sodium chloride, 0.03% magnesium sulfate, 0.03% dipotassium hydrogen phosphate [wt/vol]) containing different sole carbon sources at hourly intervals. The substrates were 2% (wt/vol) glucose, 2% (wt/vol) acetate (pH 6.0), 2% (wt/vol) oxalate (pH 6.0), 2% (wt/vol) citrate (pH 6.0), 2% (wt/vol) lactate (pH 6.0), 2% (wt/vol) pyruvate (pH 6.0), 2% (wt/vol) pyruvate (pH 6.0) plus 2% (wt/vol) glucose, 2% (wt/vol) cicada slough, and 2% (wt/vol) cicada slough plus 2% (wt/vol) glucose. Standard procedures for DNA manipulations were followed (43). Escherichia coli strain DH5α was used for routine bacterial transformations and maintenance of plasmids.
Plasmid construction.
Plasmids pBT and pBFT contained the herbicide resistance gene bar and the herbicide resistance protein and enhanced green fluorescent protein (GFP) fusion gene bar::egfp, respectively.
On the basis of pBT, a vector containing the BbJEN1p::gus cassette was constructed. BbJEN1p (the BbJEN1 promoter) and gus were amplified from the genomic DNA of B. bassiana and pBANF-bar-pAN-GUS (21), respectively, using primers P1 and P2 and primers P3 and P4, respectively (Table 1). The resultant fragments were cloned into the pUC vector (Sangon) and sequenced. The BbJEN1 promoter, approximately 2.8 kb, was excised from the cloning vector by EcoRI and NotI and fused upstream of gus to form pUC-BbJEN1P-gus. trpCt (the trpC terminator) was recovered from pAN52-1 (21) by BamHI and XbaI digestion and ligated downstream of gus. pBT was excised by HindIII and XbaI, and a BbJEN1p::gus::trpCt fusion gene was inserted, resulting in pBT-BbJEN1P-gus.
TABLE 1.
Primers used in this work
| Primer | Sequence (5′-3′)a | Restriction enzyme site |
|---|---|---|
| P1 | GAATTCGTGATCAGTCAGGCATGT | EcoRI |
| P2 | GCGGCCGCAATTGTATGGACTATAG | NotI |
| P3 | GCGGCCGCATGTTACGTCCTGT | NotI |
| P4 | GGATCCTCATTGTTTGCCTC | BamHI |
| P5 | GCGGCCGCGTCGCCACCATGGTGAGCAA | NotI |
| P6 | GGATCCTTAGGACCTGTACAGCTCG | BamHI |
| P7 | GAATTCCGTGACG GCAAAAAGCAA | EcoRI |
| P8 | GAATTCAAGACCATGGGCCACTTG | EcoRI |
| P9 | TCTAGAGGTCTTTCCATCGCTGTT | XbaI |
| P10 | AAGCTTTGGCCTTTTCCACATCCT | HindIII |
| P11 | GCGGCCGCTCCCACAGTCATGGCCACT | NotI |
| P12 | GGATCCCAACTACTTCTTCACCTCG | BamHI |
| P13 | CCGTCAGTATCATCCAGTAAG | |
| P14 | AGTACTTAGGGGAAATAA | |
| P15 | ATGGCAATGCCATTGCCATG | |
| P16 | TTTACCTCGGAAAGCTCGAC |
Underlined sequences are restriction sites.
On the basis of pBT-BbJEN1P-gus, a vector containing the BbJEN1p::egfp cassette was constructed. The egfp gene was amplified by PCR from the plasmid pEGFP-C1 (GenBank accession number U55763; BD Biosciences Clontech, CA) by using the P5 and P6 primers (Table 1) and cloned into the pUC vector. The egfp gene was recovered from the cloning vector by NotI and BamHI digestion and inserted into pBT-BbJEN1P-gus, replacing gus to form pBT-BbJEN1P-egfp.
On the basis of pBFT, vectors overexpressing BbJEN1 and vectors disrupting BbJEN1 were constructed. To disrupt BbJEN1 in B. bassiana on the basis of homologous recombination, the herbicide resistance protein and enhanced green fluorescent protein fusion gene (bar::egfp) cassette was inserted into BbJEN1. The 5′ end of BbJEN1 was cloned by PCR with primers P7 and P8 (Table 1). The resultant PCR product was digested with EcoRI and inserted into the EcoRI site of pBFT to form pBFT-BbJEN1L. The 3′ end of BbJEN1 was amplified with primers P9 and P10 (Table 1). The PCR product was then digested with XbaI and HindIII and cloned into the corresponding sites of pBFT-BbJEN1L to form pBFT-BbJEN1L/R.
To overexpress BbJEN1 in B. bassiana, the vector overexpressing BbJEN1 was constructed. The construction of the BbJEN1 overexpression vector pBFT-BbJEN1 was conducted as follows. Primers P11 and P12 (Table 1) were used to amplify BbJEN1, using B. bassiana genomic DNA as the template. The resultant PCR product was cloned into pGEM-T (Promega) to form pGEM-BbJEN1 and sequenced for confirmation. BbJEN1 was excised from pGEM-BbJEN1 by NotI and BamHI digestion and inserted into pBFT with NotI and BamHI digestion to form pUC-PgpdA-BbJEN1-TrpC. The resultant plasmid was digested with the EcoRI restriction enzyme, and the sticky ends were made blunt using T4 DNA polymerase and digested with XbaI. The 4.7-kb cleaved DNA fragment was recovered and inserted into pBFT to form pBFT-BbJEN1.
Fungal transformation and transformant screening.
Fungal transformations were conducted as previously described (30). Genomic DNA of the B. bassiana wild-type strain and the transformants was extracted according to a previously described method (41). PCR analysis was performed to screen the pBT-BbJEN1P-gus, pBT-BbJEN1P-egfp, and BbJEN1-disrupted transformants by using primers P3 and P4, P5 and P6, and P13 and P14, respectively. Southern blot analysis was used to confirm disruption of BbJEN1 in B. bassiana transformants. Reverse transcription-PCR (RT-PCR) analysis was performed to screen BbJEN1 overexpression transformants by using primers P15 and P16. Northern blot analysis was used to confirm overexpression of BbJEN1 in B. bassiana transformants.
Determination of conidial yield.
Conidial yield was determined using the method described by Fang et al. (21). The conidia were cultivated on plates with modified Czapek's medium containing 0.5% (wt/vol) sucrose.
Measurement of carboxylate content in hyphae.
To measure the carboxylates, hyphae were incubated for 24 h in volumes of minimal medium containing various carboxylates as sole carbon sources and harvested to be lyophilized. The lyophiled hyphae were flash frozen in liquid nitrogen and comminuted with a mortar and pestle. Samples (0.2 g [dry weight]) were transferred to 4.5 ml of distilled water and mixed well. After being kept on ice for 10 min, the samples were centrifuged at 13,000 × g for 10 min. Five hundred microliters of supernatant was used to measure the carboxylates with the P/ACE MDQ capillary electrophoresis system (Beckman & Coulter). Standard samples were from Fluka.
β-Glucuronidase activity assay.
β-Glucuronidase activity was measured as described previously (29). The protein content was determined with a previously described method (5).
Microscopy.
To produce hyphae for microscopic analysis, the BbJEN1p::egfp strain was incubated in SDY medium for about 48 h, transferred to volumes of minimal medium containing different substrates, and cultivated for 12 h to detect fluorescence. For microscopic analysis, adult aphids (Myzus persicae) and cabbage worms (Pieris rapae) were inoculated with fungal conidia of the BbJEN1p::egfp strain. Infected insects were embedded by embedding medium (Tissue-Tek; Sakura), frozen to ensure optimal cutting temperature (−28°C), and sectioned (15 μm) on a freezing microtome (Leica CM1900, Germany). The infected aphids and the tissue of insects were directly mounted onto slides and sequentially imaged in the same microscopic field of light and fluorescence. Samples were observed and photographed with an Olympus model BX41 fluorescence microscope (Olympus, Tokyo, Japan) and an Olympus model MVX10 fluorescence stereomicroscope (Olympus, Tokyo, Japan). Both microscopes were equipped for fluorescence with a mercury lamp, with an excitation filter of 460 to 480 nm and a barrier filter of 495 to 540 nm. Light and fluorescent images were captured with a charge-coupled-device (CCD) camera (Olympus model DP71, Tokyo, Japan) connected to a computer.
Bioassay.
To test the virulence of fungal strains, adult aphids (M. persicae) were used for the bioassay. The bioassay was conducted as previously described (18). Fifty percent lethal concentrations (LC50s), confidence intervals, and values for other regression parameters were determined using the DPS program.
pH determinations for infected insect cuticles.
pH determinations for infected insect cuticles were conducted as previously described (47). Cuticles from third-instar P. rapae worms were applied.
RESULTS
Molecular cloning and biochemical function validation of BbJEN1.
Previously, a mutant, designated T12, characterized by a higher conidial yield than that obtained with the wild-type strain, had been identified in the pool of B. bassiana T-DNA insertional mutants constructed by the Agrobacterium-mediated-transformation method (21). On the basis of the flanking sequence of T12, a 4,500-bp DNA fragment was cloned and sequenced. The fragment contained the full-length carboxylic transport protein gene, named BbJEN1 (GenBank accession number AY187630), as well as upstream and downstream regulatory sequences. The predicted protein of BbJEN1 contains 11 transmembrane domains and was homologous to the JEN1 protein from Metarhizium anisopliae, with 77% similarity. Southern blotting showed that BbJEN1 existed as a single-copy gene in the B. bassiana genome (data not shown).
To determine the expression profile and the function of BbJEN1, a series of vectors were constructed (Fig. 1). The strategies of targeted gene disruption and overexpression were used to study the function of BbJEN1. For gene disruption, the fusion gene cassette of the herbicide resistance gene (bar) and the enhanced green fluorescent protein gene (egfp) was employed as a visible and selectable marker and inserted into BbJEN1 (Fig. 2A). Homologous and ectopic recombination and integration numbers were determined by Southern blot analysis (Fig. 2B). For overexpression of BbJEN1, the gene was placed downstream of a constitutive promoter, gpdAp from Aspergillus nidulans, and the gene cassette was delivered into B. bassiana by the electroporation method. Fifty herbicide-resistant colonies were obtained and analyzed. Northern blot analysis showed that BbJEN1 was strongly expressed in transgenic strains holding the gpdAp::BbJEN1 cassette (Fig. 3).
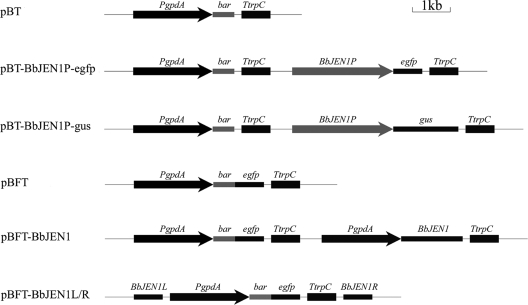
FIG. 1.
Maps of pBT, pBT-BbJEN1P-egfp, pBT-BbJEN1P-gus, pBFT, the overexpression vector for BbJEN1 (pBFT-BbJEN1), and the disruption vector for BbJEN1 (pBFT-BbJEN1L/R). PgpdA is the promoter of gpd from Aspergillus nidulans, bar is the herbicide resistance gene, egfp is the enhanced green fluorescent protein gene, gus is the β-glucuronidase gene, TtrpC is the terminator of trpC from A. nidulans, BbJEN1 is the carboxylate transport protein gene, and BbJEN1p is the promoter of BbJEN1 gene in B. bassiana.
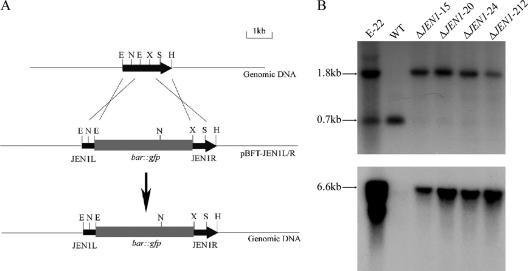
FIG. 2.
Targeted disruption of the BbJEN1 locus. (A) BbJEN1 locus and disruption vector (pBFT-JEN1L/R). pBFT-JEN1L/R, containing the bar::egfp cassette, was digested with HindIII and purified by gel electrophoresis to transform B. bassiana Bb0062 conidia. (B) DNA gel blot analysis of BbJEN1-disrupted strains. Genomic DNA was digested with NcoI/SmaI (up) and DraI (down). The 550-bp BbJEN1 fragment (up) and the 500-bp bar fragment (down) were labeled with [α-32P]dCTP and used as a hybridization probe. E-22 is an ectopic transformant. “WT” represents the wild-type strain. ΔJEN1-15, ΔJEN1-20, ΔJEN1-24, and ΔJEN1-212 are BbJEN1-disrupted strains.
FIG. 3.
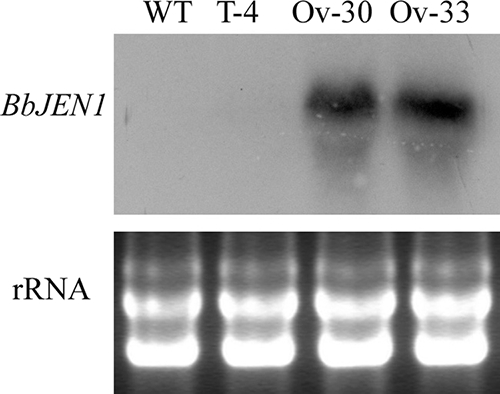
Overexpression of BbJEN1 in B. bassiana. Northern blot analysis was performed by probing total RNA of the B. bassiana wild-type strain and transformant hyphae grown in SDY (containing 4% [wt/vol] glucose to repress the expression of the native BbJEN1 gene) with the 550-bp BbJEN1 fragment labeled by [α-32P]dCTP. Each lane represents about 30 μg of total RNA. The bottom panel shows the loading controls, where each lane represents 20 μg of total RNA. “WT” represents the wild-type strain. T-4 is a transgenic control. Ov-30 and Ov-33 are BbJEN1 overexpression strains.
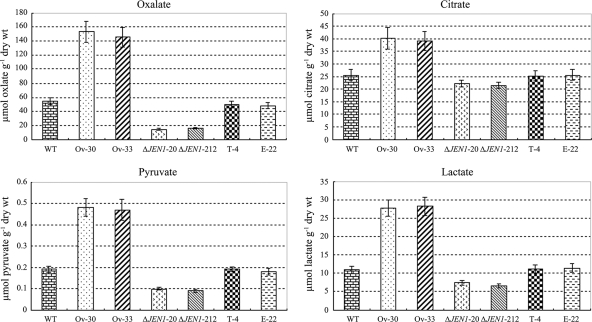
To determine the biochemical role of BbJEN1 in the transport of carboxylates, the oxalate, citrate, pyruvate, and lactate contents in hyphae of BbJEN1-disrupted strains, BbJEN1 overexpression strains, and the wild-type strain were measured by capillary electrophoresis. The amounts of the carboxylates investigated were significantly decreased in BbJEN1 disruption hyphae, whereas they were increased greatly in BbJEN1 overexpression hyphae. Measurements of carboxylates demonstrated approximately 2-fold-increased levels of oxalate and pyruvate in BbJEN1 overexpression strains in comparison to the levels for the wild-type strain, the transgenic control (T-4, holding an empty vector), and the ectopic transformant (E-22), while these two carboxylates in BbJEN1-disrupted strains were decreased about 1- to 2-fold over the levels for the controls. Likewise, lactate and citrate were increased in BbJEN1 overexpression strains and were decreased in BbJEN1-disrupted strains to obvious degrees (Fig. 4). The results indicated that BbJEN1 was a functional carboxylate transport protein gene in B. bassiana.
FIG. 4.
BbJEN1 could transport the carboxylates in B. bassiana. Hyphae were cultivated for about 2 days in SDY medium and then harvested and transferred to volumes of minimal medium supplemented with different carboxylates at concentrations of 0.2% and were incubated for 24 h. The amounts of carboxylates in hyphae were measured with capillary electrophoresis. “WT” represents the wild-type strain. T-4 is a transgenic control. Ov-30 and Ov-33 are BbJEN1-overexpression strains. E-22 is an ectopic transformant. ΔJEN1-20 and ΔJEN1-212 are BbJEN1-disrupted strains.
BbJEN1 influenced conidial yield of B. bassiana.
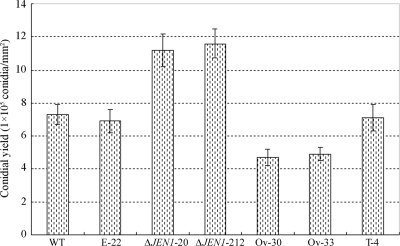
BbJEN1 was cloned by tagging a T-DNA insertional mutant, T12, one phenotype of which showed an increase in conidial yield. When BbJEN1 was disrupted through the homologous recombination method, the same phenomenon was observed. The gene-disrupted strains, ΔJEN1-20 and ΔJEN1-212, showed around 60% more conidia than the wild type. On the other hand, overexpression of the gene resulted in a significant decrease in conidial yield. Meanwhile, no obvious difference in conidial yield was found between the wild type, the transgenic control, and the ectopic transformant (Fig. 5). These results demonstrated that the expression level of BbJEN1 influenced the conidial yield of B. bassiana.
FIG. 5.
BbJEN1 influenced conidial yield in B. bassiana. “WT” represents the wild-type strain. T-4 is a transgenic control. Ov-30 and Ov-33 are BbJEN1 overexpression strains. E-22 is an ectopic transformant. ΔJEN1-20 and ΔJEN1-212 are BbJEN1-disrupted strains. The conidia were cultivated on plates with modified Czapek's medium containing 0.5% (wt/vol) sucrose.
BbJEN1 expression was induced by carboxylates and the insect cuticle and was repressed by glucose.
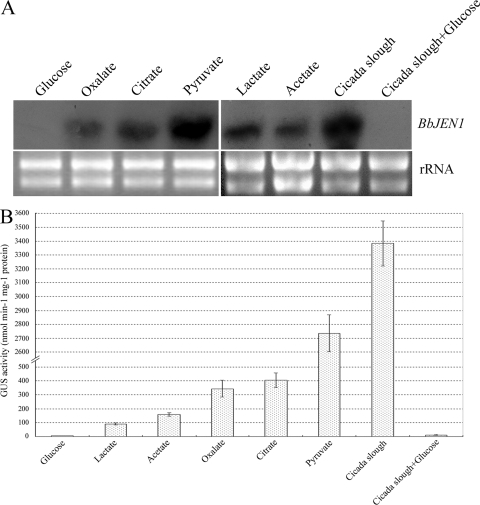
Northern blot analysis was used to investigate the induction pattern of BbJEN1 transcription of B. bassiana. Hyphae of the wild-type strain were cultured in minimal medium containing a carboxylate (lactate, acetate, oxalate, citrate, or pyruvate), glucose, or cicada slough. All carboxylates tested could induce gene expression. However, the levels inducible by various carboxylates were different. Pyruvate induced gene expression strongly. The transcript levels of BbJEN1 in the hyphae grown in pyruvate-containing medium were about 5-fold, 3-fold, 6-fold, and 8-fold higher than those in the oxalate-, citrate-, lactate-, and acetate-grown hyphae, respectively. Interestingly, BbJEN1 transcript level was greatly induced by cicada slough, with a 1.5-fold increase over the level for pyruvate. However, the transcript was not detectable in hyphae grown in glucose-containing medium, even when cicada slough was added (Fig. 6A).
FIG. 6.
BbJEN1 expression was induced by carboxylates and cicada slough and repressed by glucose. (A) Northern analysis of BbJEN1 transcripts from the wild-type strain induced by various substrates. Hyphae were cultivated for 48 h in SDY and then harvested, washed, and transferred to volumes of minimal medium supplemented with various substrates at concentrations of 0.2%. Total RNA was extracted at 12 h after the shift. Each lane contained about 30 μg of total RNA. Hybridization was carried out using a BbJEN1 fragment labeled by [α-32P]dCTP. Both bottom panels represent the loading controls, where each lane represents 20 μg of total RNA. (B) Analysis of GUS activity from the transgenic BbJEN1p::gus strain induced by various substrates. The hyphae of the BbJEN1p::gus strain were cultivated for 48 h in SDY and transferred to volumes of minimal medium culture containing various substrates at concentrations of 0.2%. After being cultivated for 12 h, hyphae were harvested and the GUS activity of the hyphae was measured.
To confirm the induction pattern of the gene, a 2.8-kb promoter sequence of BbJEN1 was fused to gus and egfp. Transgenic fungi with gene cassettes of BbJEN1p::gus or BbJEN1p::egfp were generated. The fungi holding BbJEN1p::gus were cultured in minimal medium containing glucose, acetate, oxalate, citrate, lactate, pyruvate, cicada slough, or cicada slough plus glucose. In all transgenic hyphae, GUS levels reached the peak value at 12 h. The highest GUS activity level was detected in the hyphae grown in medium containing cicada slough, with a 1.3-fold increase over the second-highest activity level, which was induced by pyruvate (Fig. 6B).
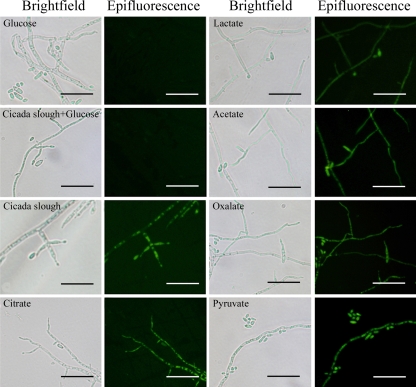
To further investigate the induction pattern, the hyphae and conidia of fungi holding BbJEN1p::egfp grown in volumes of minimal medium containing different substrates were observed using a microscope. Fluorescence was detected in the fungi grown in medium supplemented with a carboxylate or cicada slough, while no fluorescence was observed when glucose was present (Fig. 7). These results were consistent with those observed in the Northern blot pattern and the GUS pattern. Taken together, these data indicate that BbJEN1 expression was induced by the insect cuticle and carboxylates but suppressed by glucose.
FIG. 7.
GFP fluorescence was induced by carboxylates and cicada slough and repressed by glucose in the BbJEN1p::egfp line. Hyphae were cultivated for 48 h in SDY and transferred to volumes of minimal medium containing various substrates. After being cultivated for 12 h, hyphae were observed with epifluorescence or bright-field microscopy. Bars = 25 μm.
BbJEN1 expressed in the hyphae penetrating the cuticle and growing on the cadaver.
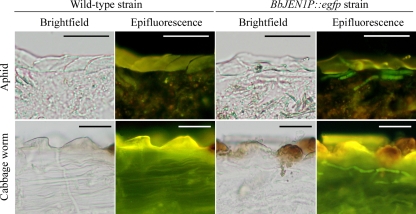
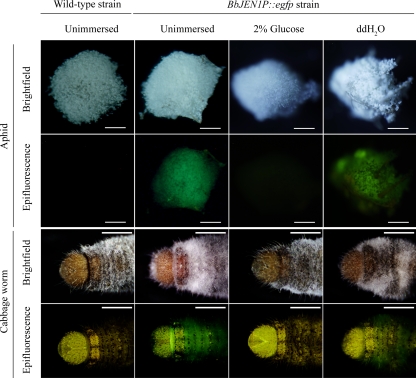
To reveal BbJEN1 expression during infection, aphids (M. persicae) and cabbage worms (P. rapae) were inoculated with conidial suspension (1 × 107 conidia ml−1) of transgenic B. bassiana holding the BbJEN1p::egfp cassette. The infected insects were sectioned (15 μm) to observe fluorescence of GFP of hyphae in insect tissues. During infection, fluorescence in the hyphae in the penetrated cuticle was observed, and no fluorescence was detected in the hyphae of the wild-type strain (Fig. 8). No GFP activity was observed in the hyphal bodies in the hemolymph of the host infected by fungi holding BbJEN1p::egfp, indicating that the BbJEN1 promoter directed the expression of the GFP gene during the penetration process. Furthermore, fluorescence was also obvious in the hyphae growing on the cadavers of green peach aphids (M. persicae) and cabbage worms (P. rapae) after the demise of the host, which resulted from the profuse growth of the fungi in the hemolymph. However, GFP activity was significantly inhibited when the cadavers were treated with glucose (Fig. 9).
FIG. 8.
BbJEN1 expressed during penetration of the insect cuticles. Green peach aphids (M. persicae) and cabbage worms (P. rapae) were inoculated with the wild-type strain and the BbJEN1p::egfp transformant. After 48 h, the infected insects were sliced into 15-μm sections with a Leica microtome and observed with epifluorescence or bright-field microscopy. Bars = 25 μm.
FIG. 9.
BbJEN1 expressed in the hyphae growing on the cadaver. Green peach aphids (M. persicae) and cabbage worms (P. rapae) were inoculated with the wild-type strain and the BbJEN1p::egfp transformant, respectively. After 7 days, some infected insects were immersed in 2% (wt/vol) glucose and distilled water for 3 h. The infected insects were mounted onto slides to detect the fluorescence of the hyphae under a fluorescence microscope. The bars correspond to 1 mm (aphid) or 2 mm (cabbage worm).
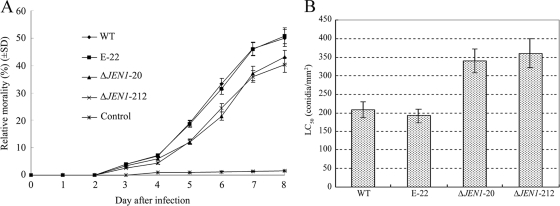
BbJEN1 disruption reduced the virulence of B. bassiana.
The fact that BbJEN1 was strongly induced by the insect cuticle and highly expressed in the penetration process implies that BbJEN1 may affect the virulence of the fungi. To test if deleting BbJEN1 influences fungal virulence, we bioassayed adult aphids (M. persicae) treated with gene disruption strains and controls. Aphids were inoculated with fungal conidia at a concentration of 5 × 106 conidia ml−1. The relative mortality rates among adult aphids were significantly reduced with BbJEN1-disrupted strains ΔJEN1-20 (43.11%) and ΔJEN1-212 (40.32%) in comparison with the levels for the wild type (50.05%) and the ectopic transformant E-22 (50.83%) at 8 days after inoculation (Fig. 10A). Meanwhile, the LC50s (concentrations needed for the pathogen to kill 50% of aphids) of ΔJEN1-20 and ΔJEN1-212 were nearly 1-fold higher than those of the wild-type strain and the ectopic transformant (E-22) (Tukey's post hoc tests; F3,8 = 27.426; P < 0.01) (Fig. 10B). The LC50 was based on the efficiency of infection (54). The increases in LC50 and the decreases in mortality rate induced by the mutants suggested that disruption of BbJEN1 impaired the infection efficiency of B. bassiana in aphids. However, overexpression of BbJEN1 induced no significant change in the virulence of the pathogen (data not shown).
FIG. 10.
BbJEN1 disruption reduced the virulence of the fungi. (A) Relative mortality rates among adult aphids (M. persicae). Aphids were inoculated with fungal conidia at a concentration of 5 × 106 conidia ml−1 by using a Potter precision laboratory spray tower (Burkard Manufacturing Co., Ltd., England). Control aphids were treated with water plus 0.05% (vol/vol) Tween 80. (B) LC50s of BbJEN1-disrupted strains and the wild-type strain for M. persicae at 7 days after inoculation. Aphids were inoculated with fungal conidia by using a Potter precision laboratory spray tower (Burkard Manufacturing Co., Ltd., England). Five conidial suspensions (5 × 105, 1 × 106, 5 ×106, 1 × 107, and 5 × 107 conidia ml−1) were used for inoculation.
Ambient pH influences the virulence of several pathogenic fungal species during infection (7, 31, 32, 47). To illustrate the possible connection between BbJEN1 expression and the ambient pH values of insect cuticles, we measured the pH values in cuticles of third-instar P. rapae worms infected by the BbJEN1-disrupted strain, the BbJEN1 overexpression strain, and the wild-type strain. The natural pH in the P. rapae cuticle was 6.50 ± 0.05. After infection with the wild-type strain, the pH in cuticles rose to 7.82 ± 0.06. Interestingly, the pH in the cuticle infected by the BbJEN1-disrupted strain was lower (7.65 ± 0.03), while the value in the cuticle infected by BbJEN1 overexpression strain was higher (8.02 ± 0.05) than that in the cuticle infected by the wild-type strain (7.82 ± 0.06) (Tukey's post hoc tests; F2,6 = 44.1; P < 0.01). It was deduced that BbJEN1 disruption decreased the transfer of organic acid from the cuticle into cells of the fungi, resulting in lower ambient pH values in infected cuticles, and consequently, the virulence of the fungi was impaired.
DISCUSSION
The current work demonstrated that BbJEN1 encodes a functional JEN1 protein, which transports carboxylates into cells. In yeast, JEN1p is an important transporter of carboxylates (2, 9, 45). We measured the amounts of carboxylates in hyphae of BbJEN1-disrupted and BbJEN1 overexpression strains and demonstrated that BbJEN1p could transport not only monocarboxylates but also some dicarboxylates and tricarboxylates into B. bassiana.
BbJEN1 was cloned through tagging of the T12 mutant, which produced more conidia than the wild type (21). When BbJEN1 was disrupted through the homologous recombination method, the same phenomenon was observed, whereas when the gene was overexpressed, the conidial yield was significantly decreased, suggesting that the conidial yield is relative to BbJEN1 expression. For fungi, the carbon source is considered an important nutrition factor for conidial yield (28, 42, 44). BbJEN1p can transfer carboxylates into cells. Disruption of carboxylate transport may affect conidial yield through nutritional alteration.
Northern analysis showed that BbJEN1 was strongly induced by cicada slough. To investigate the expression pattern of BbJEN1 during infection of the fungi, we employed GFP as a marker controlled by the BbJEN1 promoter and monitored the marker protein activities. It was demonstrated that BbJEN1p::egfp was highly expressed during the penetration of the insect cuticles by the fungi. However, we had not found BbJEN1p::egfp expression in the hemolymph of the cabbage worm. This implies that BbJEN1 may be involved in infection and that its expression level may affect the virulence of the fungi.
To test this hypothesis, we performed the bioassay by infecting aphids with BbJEN1-disrupted fungi. Significant reductions in relative mortality rate among adult aphids and significant increases in LC50 were observed. The LC50 increase reflects the decrease in infection efficiency (54). The LC50 increases obtained with BbJEN1-disrupted strains demonstrated that BbJEN1 disruption reduced the virulence of B. bassiana to insects. Why does BbJEN1 disruption decrease the virulence of the fungi? An explanation is that BbJEN1 disruption impairs the importation of organic acids, such as oxalic acid, produced during penetration of the insect cuticles, into fungal cells, resulting in alteration of the ambient pH condition during invasion. Extracellular pH is considered a major determinant in expression of genes essential to the growth, differentiation, and virulence in fungal pathogens of humans, plants, and insects (22, 37, 40, 47, 55, 56). Gene expression in fungi induced by ambient pH is regulated via a conserved signaling cascade (3, 4, 27, 32, 39). Among the genes controlled by ambient pH are those encoding extracellular enzymes (31, 36, 47), components of secondary metabolite biosynthetic pathways (6, 16), and cell wall biosynthesis proteins (8, 38). Some of these pH-regulated genes have been implicated in fungal pathogenesis. Alkalinization of the insect cuticle, possibly by the fungus itself, is a physiological signal that triggers the production of pathogenicity factors (12), and alkalinization of the cuticle during infection increased cuticle-degrading protease production (48). It is reported that the transport of acids would change the extracellular acid-base equilibrium (10). BbJEN1p helps the fungi in transporting carboxylates into cells. Consequently, a decrease of extracellular organic acids will lead to alkalinization of the infected cuticles. Thus, when BbJEN1 was disrupted, the pH value was lowered by the accumulated organic acids in the infected cuticles, and the lower pH may result in impairment of the virulence of the pathogen.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of China (2009CB118904 to Y. Pei), the Hi-Tech Research and Development Program of China (2006AA10A212 to Y. Pei), and the Natural Science Foundation of China (30300235 to Y. Zhang).
Footnotes
Published ahead of print on 23 October 2009.
REFERENCES
- 1.Akita, O., C. Nishimori, T. Shimamoto, T. Fujii, and H. Iefuji. 2000. Transport of pyruvate in Saccharomyces cerevisiae and cloning of the gene encoded pyruvate permease. Biosci. Biotechnol. Biochem. 64:980-984. [DOI] [PubMed] [Google Scholar]
- 2.Andrade, R. P., and M. Casal. 2001. Expression of the lactate permease gene JEN1 from the yeast Saccharomyces cerevisiae. Fungal Genet. Biol. 32:105-111. [DOI] [PubMed] [Google Scholar]
- 3.Aréchiga-Carvajal, E. T., and J. Ruiz-Herrera. 2005. The RIM101/pacC homologue from the basidiomycete Ustilago maydis is functional in multiple pH-sensitive phenomena. Eukaryot. Cell 4:999-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bignell, E., S. Negrete-Urtasun, A. M. Calcagno, K. Haynes, H. N. Arst, and T. Rogers. 2005. The Aspergillus pH-responsive transcription factor PacC regulates virulence. Mol. Microbiol. 55:1072-1084. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method after the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Brakhage, A. A., P. Spröte, Q. Al-Abdallah, A. Gehrke, H. Plattner, and A. Tüncher. 2004. Regulation of penicillin biosynthesis in filamentous fungi. Adv. Biochem. Eng. Biotechnol. 88:45-90. [DOI] [PubMed] [Google Scholar]
- 7.Caddick, M. X., A. G. Brownlee, and H. N. Arst. 1986. Regulation of gene expression by pH of the growth medium in Aspergillus nidulans. Mol. Gen. Genet. 203:346-353. [DOI] [PubMed] [Google Scholar]
- 8.Caracuel, Z., A. L. Martínez-Rocha, A. di Pietro, M. P. Madrid, and M. I. Roncero. 2005. Fusarium oxysporum gas1 encodes a putative beta-1, 3-glucanosyltransferase required for virulence on tomato plants. Mol. Plant-Microbe Interact. 18:1140-1147. [DOI] [PubMed] [Google Scholar]
- 9.Casal, M., S. Paiva, R. P. Andrade, C. Gancedo, and C. Leão. 1999. The lactate-proton symport of Saccharomyces cerevisiae is encoded by JEN1. J. Bacteriol. 181:2620-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cássio, F., M. Côrte-Real, and C. Leão. 1993. Quantitative analysis of proton movements associated with the uptake of weak carboxylic acids: the yeast Candida utilis as a model. Biochim. Biophys. Acta 1153:59-66. [DOI] [PubMed] [Google Scholar]
- 11.Charnley, A. K., and R. J. St. Leger. 1991. The role of cuticle-degrading enzymes in fungal pathogenesis in insects, p. 267-287. In E. T. Cole and H. C. Hoch (ed.), Fungal spore disease initiation in plants and animals. Plenum Press, New York, NY.
- 12.Charnley, A. K. 2003. Fungal pathogens of insects: cuticle degrading enzymes and toxins. Adv. Bot. Res. 40:241-321. [Google Scholar]
- 13.Cho, E. M., D. Boucias, and N. O. Keyhani. 2006. EST analysis of cDNA libraries from the entomopathogenic fungus Beauveria (Cordyceps) bassiana. II. Fungal cells sporulating on chitin and producing oosporein. Microbiology 152:2855-2864. [DOI] [PubMed] [Google Scholar]
- 14.Cho, E. M., L. Liu, W. Farmerie, and N. O. Keyhani. 2006. EST analysis of cDNA libraries from the entomopathogenic fungus Beauveria (Cordyceps) bassiana. I. Evidence for stage-specific gene expression in aerial conidia, in vitro blastspores and submerged conidia. Microbiology 152:2843-2854. [DOI] [PubMed] [Google Scholar]
- 15.Clarkson, J. M., and A. K. Charnley. 1996. New insights into the mechanisms of fungal pathogenesis in insects. Trends Microbiol. 4:197-203. [DOI] [PubMed] [Google Scholar]
- 16.Eisendle, M., H. Oberegger, R. Buttinger, P. Illmer, and H. Haas. 2004. Biosynthesis and uptake of siderophores is controlled by the PacC-mediated ambient-pH regulatory system in Aspergillus nidulans. Eukaryot. Cell 3:561-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enerson, B. E., and L. R. Drewes. 2003. Molecular features, regulation and function of monocarboxylate transporters: implications for drug delivery. J. Pharm. Sci. 92:1531-1544. [DOI] [PubMed] [Google Scholar]
- 18.Fan, Y., W. Fang, S. Guo, X. Pei, Y. Zhang, Y. Xiao, D. Li, K. Jin, M. J. Bidocjka, and Y. Pei. 2007. Increased insect virulence in Beauveria bassiana strains overexpressing an engineered chitinase. Appl. Environ. Microbiol. 73:295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang, W., B. Leng, Y. Xiao, K. Jin, J. Ma, Y. Fan, J. Feng, X. Yang, Y. Zhang, and Y. Pei. 2005. Cloning of Beauveria bassiana chitinase gene Bbchit1 and its application to improve fungal strain virulence. Appl. Environ. Microbiol. 71:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang, W., M. Pava-ripoll, S. Wang, and R. J. St. Leger. 2009. Protein kinase A regulates production of virulence determinants by the entomopathogenic fungus, Metarhizium anisopliae. Fungal Genet. Biol. 46:277-285. [DOI] [PubMed] [Google Scholar]
- 21.Fang, W., Y. Zhang, X. Zheng, X. Yang, H. Duan, Y. Li, and Y. Pei. 2004. Agrobacterium tumefaciens-mediated transformation of Beauveria bassiana using an herbicide resistance gene as a selection marker. J. Invertebr. Pathol. 85:18-24. [DOI] [PubMed] [Google Scholar]
- 22.Freitas, J. S., E. M. Silva, and A. Rossi. 2007. Identification of nutrient-dependent changes in extracellular pH and acid phosphatase secretion in Aspergillus nidulans. Genet. Mol. Res. 6:721-729. [PubMed] [Google Scholar]
- 23.Guiard, B. 1985. Structure, expression and regulation of a nuclear gene encoding a mitochondrial protein: the yeast L(+)-lactate cytochrome c oxidoreductase (cytochrome b2). EMBO J. 4:3265-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajek, A. E., and R. J. St. Leger. 1994. Interactions between fungal pathogenesis and insect hosts. Annu. Rev. Entomol. 39:293-322. [Google Scholar]
- 25.Halestrap, A. P., and D. Meredith. 2004. The SLC16 gene familiy-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 447:619-628. [DOI] [PubMed] [Google Scholar]
- 26.Halestrap, A. P., and N. T. Price. 1999. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem. J. 343:281-299. [PMC free article] [PubMed] [Google Scholar]
- 27.Hervás-Aguilar, A., J. M. Rodríguez, J. Tilburn, H. N. Arst, and M. A. Peñalva. 2007. Evidence for the direct involvement of the proteasome in the proteolytic processing of the Aspergillus nidulans zinc finger transcription factor PacC. J. Biol. Chem. 282:34735-34747. [DOI] [PubMed] [Google Scholar]
- 28.Jackson, M. A., and D. A. Schisler. 1992. The composition and attributes of Colletotrichum truncatum spores are altered by the nutritional environment. Appl. Environ. Microbiol. 58:2260-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jefferson, R. A. 1987. Assaying chimeric genes in plants from gene fusion system. Plant Mol. Biol. Rep. 5:387-405. [Google Scholar]
- 30.Jin, K., Y. Zhang, Z. Luo, Y. Xiao, Y. Fan, D. Wu, and Y. Pei. 2008. An improved method for Beauveria bassiana transformation using phosphinothricin acetlytransferase and green fluorescent protein fusion gene as a selectable and visible marker. Biotechnol. Lett. 30:1379-1383. [DOI] [PubMed] [Google Scholar]
- 31.Kanda, S., T. Aimi, S. Kano, S. Ishihara, Y. Kitamoto, and T. Morinaga. 2008. Ambient pH signaling regulates expression of the serine protease gene (spr1) in pine wilt nematode-trapping fungus, Monacrosporium megalosporum. Microbiol. Res. 163:63-72. [DOI] [PubMed] [Google Scholar]
- 32.Kullas, A. L., S. J. Martin, and D. Davis. 2007. Adaptation to environmental pH: integrating the Rim101 and calcineurin signal transduction pathways. Mol. Microbiol. 66:858-871. [DOI] [PubMed] [Google Scholar]
- 33.Lodi, T., and I. Ferrero. 1993. Isolation of the DLD1 gene of Saccharomyces cerevisiae encoding the mitochondrial enzyme d-lactate ferricytochrome c oxidoreductase. Mol. Gen. Genet. 238:315-324. [DOI] [PubMed] [Google Scholar]
- 34.Lodi, T., F. Fontanesi, and B. Guiard. 2002. Co-ordinate regulation of lactate metabolism genes in yeast: the role of the lactate permease gene JEN1. Mol. Genet. Genomics 266:838-847. [DOI] [PubMed] [Google Scholar]
- 35.Lodi, T., F. Fontanesi, I. Ferrero, and C. Donnini. 2004. Carboxylic acids permeases in yeast: two genes in Kluyveromyces lactis. Gene 339:111-119. [DOI] [PubMed] [Google Scholar]
- 36.Miyara, I., H. Shafran, H. K. Haimovich, J. Rollins, A. Sherman, and D. Prusky. 2008. Multi-factor regulation of pectate lyase secretion by Colletotrichum gloeosporioides pathogenic on avocado fruits. Mol. Plant Pathol. 9:281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno-Mateos, M. A., J. Delgado-Jarana, A. C. Codón, and T. Benítez. 2007. pH and PacI control development and antifungal activity in Trichoderma harzianum. Fungal Genet. Biol. 44:1355-1367. [DOI] [PubMed] [Google Scholar]
- 38.Nobile, C. J., N. Solis, C. L. Myers, A. J. Fay, J. S. Deneault, A. Nantel, A. P. Mitchell, and S. G. Filler. 2008. Candida albicans transcription factor Rim101 mediates pathogenic interactions through cell wall functions. Cell. Microbiol. 10:2180-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peñalva, M. A., and H. N. Arst. 2002. Regulation of gene expression by ambient pH in filamentous fungi and yeasts. Microbiol. Mol. Biol. Rev. 66:426-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peñalva, M. A., J. Tilburn, E. Bignell, and H. N. Arst. 2008. Ambient pH gene regulation in fungi: making connections. Trends Microbiol. 16:291-300. [DOI] [PubMed] [Google Scholar]
- 41.Reader, U., and P. Broda. 1985. Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol. 1:17-20. [Google Scholar]
- 42.Safavi, S. A., F. A. Shah, A. K. Pakdel, G. R. Rasoulian, A. R. Bandani, and T. M. Butt. 2007. Effect of nutrition on growth and virulence of the entomopathogenic fungus Beauveria bassiana. FEMS Microbiol. Lett. 270:116-123. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 44.Shah, F. A., C. S. Wang, and T. M. Butt. 2005. Nutrition influences growth and virulence of the insect-pathogenic fungus Metarhizium anisopliae. FEMS Microbiol. Lett. 251:259-266. [DOI] [PubMed] [Google Scholar]
- 45.Soares-Silva, I., S. Paiva, P. Kotter, K. D. Entian, and M. Casal. 2004. The disruption of JEN1 from Candida albicans impairs the transport of lactate. Mol. Membr. Biol. 21:403-411. [DOI] [PubMed] [Google Scholar]
- 46.St. Leger, R. J., L. Joshi, M. J. Bidochka, and D. W. Roberts. 1996. Construction of an improved mycoinsecticide overexpressing a toxic protease. Proc. Natl. Acad. Sci. U. S. A. 93:6349-6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.St. Leger, R. J., L. Joshi, and D. Roberts. 1998. Ambient pH is a major determinant in the expression of cuticle-degrading enzymes and hydrophobin by Metarhizium anisopliae. Appl. Environ. Microbiol. 64:709-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.St. Leger, R. J., J. O. Nelson, and S. E. Screen. 1999. The entomopathogenic fungus Metarhizium anisopliae alters ambient pH, allowing extracellular protease production and activity. Microbiology 145:2691-2699. [DOI] [PubMed] [Google Scholar]
- 49.Wang, C., Z. Duan, and R. J. St. Leger. 2008. The MOS1 osmosensor of Metarhizium anisopliae is required for adaptation to insect host hemolymph. Eukaryot. Cell 7:302-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, C., and R. J. St. Leger. 2005. Developmental and transcriptional responses to host and nonhost cuticles by the specific locust pathogen Metarhizium anisopliae var. acridum. Eukaryot. Cell 4:937-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, C., and R. J. St. Leger. 2006. A collagenous protective coat enables Metarhizium anisopliae to evade insect immune responses. Proc. Natl. Acad. Sci. U. S. A. 103:6647-6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, C., and R. J. St. Leger. 2007. The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesin enables attachment to plants. Eukaryot. Cell 6:808-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, C., and R. J. St. Leger. 2007. The Metarhizium anisopliae perilipin homolog MPL1 regulates lipid metabolism, appressorial turgor pressure, and virulence. J. Biol. Chem. 282:21110-21115. [DOI] [PubMed] [Google Scholar]
- 54.Wood, H. A. 1995. Development and testing of genetically improved baculovirus insecticides, p. 91-102. In M. L. Shuler, H. A. Wood, R. R. Granados, and D. A. Hammer (ed.), Baculovirus expression systems and biopesticides. Wiley, New York, NY.
- 55.You, B. J., M. Choquer, and K. R. Chung. 2007. The Colletotrichum acutatum gene encoding a putative pH-responsive transcription regulator is a key virulence determinant during fungal pathogenesis on citrus. Mol. Plant-Microbe Interact. 20:1149-1160. [DOI] [PubMed] [Google Scholar]
- 56.You, B. J., and K. R. Chung. 2007. Phenotypic characterization of mutants of the citrus pathogen Colletotrichum acutatum defective in a PacC-mediated pH regulatory pathway. FEMS Microbiol. Lett. 277:107-114. [DOI] [PubMed] [Google Scholar]