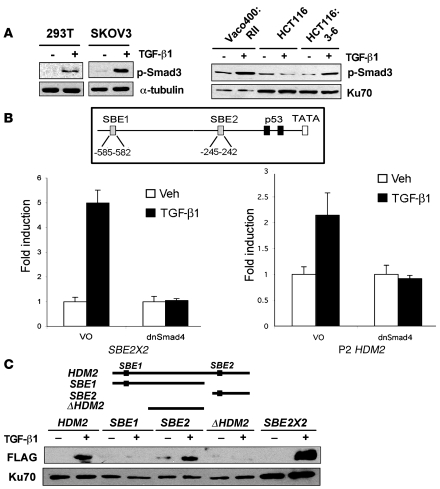
Figure 4. Smad activation and induction of the HDM2 promoter.
(A) Western blot of p-Smad3 in 293T, SKOV3, Vaco400:RII, HCT116, and HCT116:3-6 cells. Tubulin and Ku70 were used as internal controls for loading. (B) A schematic of 2 putative SBEs designated SBE1 and SBE2 and the p53-binding elements in the P2 promoter of HDM2. Reporter assays were conducted using dominant negative Smad4 (dnSmad4) or control vector only (VO) in transient transfection assays. HCT116:3-6 cells were transfected with an SBE2X2 reporter as a positive control or HDM2 P2 reporter. All samples were transfected with renilla to use as an internal control. Cells were treated with vehicle or TGF-β1 (10 ng/ml). Reporter activity was determined relative to renilla to generate relative activity. Fold induction was determined relative to vehicle control and SD was calculated relative to the mean. (C) Western blot of FLAG-Smad3 and Ku70 that bound to the HDM2 promoter. 293T cells were transfected with FLAG-Smad3 and treated with vehicle or TGF-β1 (10 ng/ml). Nuclear extracts were prepared from the latter mentioned cell treatments. Biotinylated PCR fragments of the HDM2 promoter, SBE1, SBE2, ΔHDM2, or a positive control, SBE2X2, were mixed with the nuclear extracts. Biotinylated DNA fragments bound to streptavidin beads were used to purify FLAG-Smad3 and Ku70 (internal control).