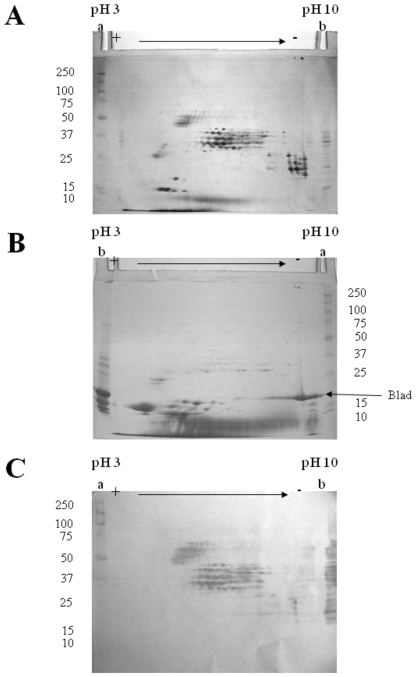
Figure 3. Two-dimensional, structural analysis of β-conglutin and blad from Lupinus albus.
β-Conglutin (A,C) and the protein containing blad (B) were extracted, purified from the cotyledons of dry seeds and eight-days germinated seedlings, respectively, and subjected to two-dimensional electrophoresis. The gels were either stained for total protein (A,B) or transferred onto a membrane and probed with anti-blad antibodies (C). Two hundred µg of protein were used to prepare the 2D-gels (A,B) and 50 µg to prepare the 2D-blot (C). Lanes a: molecular mass standards (kDa) (precision protein standards prestained, broad range, Bio-Rad, in C). Lanes b: β-conglutin (A, 50 µg; C, 15 µg) or the native protein containing blad (B, 50 µg).