Abstract
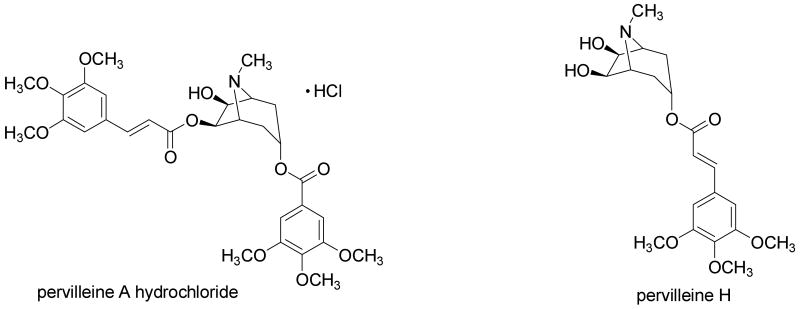
Pervilleine A is an aromatic ester tropane alkaloid from Erythroxylum pervillei that has shown promising activity as a multidrug resistance inhibitor. Due to its structural similarity with the well known (-)-hyoscyamine and (-)-cocaine, the cholinergic and adrenergic activities of pervilleine A were evaluated. At 30 μM (±)-pervilleine A hydrochloride exhibited non-competitive inhibition of the cholinergic response in the guinea pig ileum and did not affect the carbachol-induced contraction of the rat anococcygeus smooth muscle. (±)-Pervilleine A hydrochloride blocked nonspecifically the vascular response of (-)-norepinephrine in the rat aorta ring, while the contractile response of rat vas deferens to (-)-norepinephrine was not affected significantly at a 100 μM concentration. An analogue of pervilleine A, (±)-pervilleine H, without a 6-O-trans-3,4,5-trimethoxycinnamoyl ester substituent required for anti-multidrug resistance activity, did not exhibit any effects in these experiments. The data suggest that (±)-pervilleine A hydrochloride has weak nonspecific anticholinergic and vascular antiadrenergic activities. The lack of significant cholinergic and adrenergic receptor-mediated activities may be considered advantageous for the further development of pervilleine A as a new adjuvant in cancer chemotherapy.
Keywords: (±)-pervilleine A HCl, (±)-pervilleine H, tropane aromatic ester alkaloids, anticholinergic activity, antiadrenergic activity
Introduction
In previous work, bioactivity-guided fractionation of a chloroform extract of the roots of Erythroxylum pervillei Baillon (Erythroxylaceae), collected in southern Madagascar, led to the isolation of several new aromatic ester tropane alkaloids. Of these substances, pervilleines A-F were found in a tumor cell panel to reverse multidrug resistance (MDR) for the KB-V1 (vinblastine-resistant) oral epidermoid carcinoma cell line in the presence of vinblastine, while being much less cytotoxic for normal KB cells and other cancer cell lines (Silva et al., 2001). Pervilleine A was also found to restore the vinblastine sensitivity of CEM/VLB100 (multidrug-resistant human leukemic lymphoblast CEM) cells as well as the chemosensitivity to colchicine in the KB-8-5 cell line. In addition, pervilleine A was shown to be effective as an MDR inhibitory agent in an in vivo hollow fiber assay using KB-V1 cells when co-administered with vinblastine, with this tropane alkaloid postulated to act synergistically by inhibiting P-glycoprotein mediated drug efflux (Mi et al., 2001). Owing to the promising MDR-inhibitory activities of pervilleines A-C and F (Kinghorn et al., 2006), which were comparable in potency to the standard MDR inhibitor, verapamil, these compounds were selected for further development through the RAID (Rapid Access to Intervention Development) program of the U.S. National Cancer Institute. (±)-Pervilleine A (NSC 687938) is currently undergoing additional biological evaluation in a xenograft test system at the National Cancer Institute Frederick, Maryland. During a gram-scale reisolation of pervilleine A from E. pervillei stem bark, a number minor analogues were isolated, including (±)-pervilleine H (Chin et al., 2006). It has been found in a cytotoxicity-structures relationship study that in order to exhibit MDR-inhibitory activity against KB-V1 cells by pervilleine A analogues, a C-6 trans-3,4,5-trimethoxycinnamoyl ester unit must be present (Mi et al., 2002).
Since these aromatic ester tropane alkaloids are structurally similar to (-)-hysocyamine, a competitive antagonist of the actions of acetylcholine and other muscarinic agonists (Brown and Taylor, 2001), and also (-)-cocaine, which is a blocker of nerve impulses through inhibition of local norepinephrine reuptake (Catterall and Mackie, 2001), evaluations of cholinergic and adrenergic effects of (±)-pervilleine A and its analog, (±)-pervilleine H, were carried out to investigate the interactions of these compounds with these receptors of nervous system. Therefore, the pharmacological effects of (±)-pervilleines A and H were examined in in vitro cholinergic and adrenergic assay systems, using the guinea pig ileum, rat anococcygeus muscle, rat aorta, and rat vas deferens.
Materials and Methods
Test compounds
(±)-Pervilleine A hydrochloride was prepared according to the method described below. Pervilleine A (10 mg) (Silva et al., 2001) was placed in a conical reaction vial (2 mL) with a magnetic stir bar. Anhydrous ethanol (100 μL) was added into the reaction vial producing a fine suspension. A solution of concentrated ethanolic anhydrous hydrochloric acid was prepared by bubbling HCl gas into anhydrous ethanol for 20 min. This solution was slowly added to the pervilleine A in absolute ethanol until the suspension cleared and the solution was acidic (pH 2). The reaction was stirred for 15 min and then transferred into a 10 ml test tube containing anhydrous ether (15 mL). The test tube was cooled to 0 °C in an ice bath for 30 min, and a fine white precipitate formed. The test tube was placed in a sonic water bath in order to create a fine suspension. The precipitate was pelleted by centrifugation, with the supernatant solution removed using a syringe, and the precipitate washed two more times with anhydrous ether. Pervilleine A HCl exhibited mp 68-70 °C (MeOH) and [α]D 0 (c 0.07, MeOH)
(±)-Pervilleine H isolated from E. pervillei (Chin et al., 2006) was used in the free base form. (-)-Hyoscyamine hydrochloride, (-)-norepinephrine bitartrate, and carbachol were purchased from Sigma (St. Louis, MO, USA).
Animals
Male albino rats weighing 380-420 g were used for the preparations of the anococcygeus muscle, aorta rings, and vas deferens. A non-albino guinea pig (500 g) was used for the present study. All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals, U.S. National Institutes of Health.
Guinea pig ileum
A 2-cm length of guinea pig ileum, fresh or stored (24-48 h), was cleaned of its content and set up in a 10 mL organ bath containing physiological salt solution maintained at 37.5 °C (Patil, 1996). The composition of salt solution was 118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 0.57 mM MgCl2, 25 mM NaHCO3, 1.0 mM NaH2PO4, and 11.0 mM glucose, and it was prepared in double distilled water. The organ bath preparation was aerated with 95% O2 and 5% CO2. The resting tension on the ileum was 300 mg and it was allowed to equilibrate for at least 1 h.
Rat anococcygeus muscle
The left and right rat anococcygeus muscle was set up in an organ bath under the same conditions described above, under 1 g of resting tension, which dropped -0.5 g. During the experiment, a -0.5 g tension was kept constant.
Rat aorta
Rat aorta rings, 2-3 mm wide, were suspended in the same conditions as above at the resting tension of 1 gram. This vascular tissue was equilibrated for >3 h before initiation of the assay. The aorta was exposed to (-)-norepinephrine before the treatment of (±)-pervilleines A HCl and H.
Rat vas deferens
A pair of vas deferens at resting tension of 300 mg was suspended in adjacent organ bath. Each organ was washed several times for 90 min before the initiation of the experiments. The organ contractions were recorded as tension changes on the precalibrated Grass ink polygraph recorder.
The control cumulative concentration response curve (CRC) of the agonist carbachol or (-)-norepinephrine was obtained on the organ which was thoroughly washed and the CRC was repeated in the presence of the alkaloid. Depending on the organ examined, the incubation time of each alkaloid (pervilleine A HCl and pervilleine H) was either 15 or 30 min. A minimum of two CRCs and a maximum of four CRCs were obtained in each organ. The affinity constant Kβ of (-)-hyoscyamine was calculated from the equation Kβ = concentration of the blocker/the EC50 of carbachol with blocker minus the EC50 of carbachol control.
Statistical analysis
All data were expressed as means ± SEM. Significant differences between responses were analyzed by one-way analysis of variance (ANOVA) and a difference was considered as statistically significant when p < 0.05.
Results and Discussion
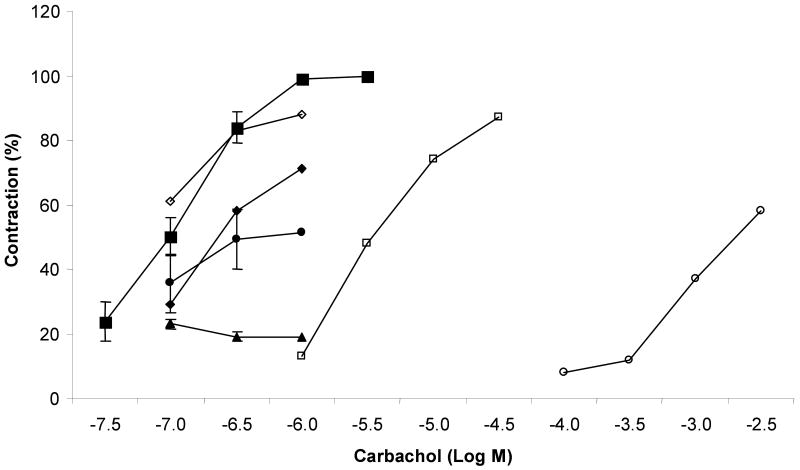
The contractile effect of carbachol, a cholinergic muscarinic agonist, on the guinea pig ileum was observed in a dose-dependent manner (EC50 = 102 nM) in Figure 1. (±)-Pervilleine A HCl (0.1 to 100 μM) exhibited an inhibitory effect against carbachol-induced contractions in the smooth muscle of ileum (Figure 1) while no significant effect of (±)-pervilleine H at 30 μM (n = 2) was obtained (data not shown). In the presence of (±)-pervilleine A HCl (30 μM), the contractile response of the ileum was reduced by 50% of the maximum effect of carbachol, and to 19% at 100 μM. In contrast, a competitive muscarinic antagonist to carbachol, (-)-hyoscyamine (0.1 to 10 μM), shifted the CRC of the agonist to the right without a decrease in the maximum response of carbachol. The affinity constant Kβ of (-)-hyoscyamine was found to be 1.8 nM, in good agreement with the literature (Patil, 1996). These results suggested that the effect of (±)-pervilleine A HCl on the ileum was not related to a competitive blocking of the muscarinic acetylcholine receptor.
Figure 1.
Concentration response curves of the muscarinic agonist, carbachol, on the guinea pig ileum in control (■) or in the presence of (±)-pervilleine A HCl at concentration of 0.1 μM (◊), 10 μM (◆), 30 μM (●) and 100 μM (▲), and in the presence of (-)-hyoscyamine at concentration of 0.1 μM (□) and 10 μM (○). For carbachol control, 9 observations from different experiments were averaged. Vertical lines are SEM. In other observation, n varied from 1 to 3.
Treatment of (±)-pervilleine A HCl (n = 2) and pervilleine H (n = 1) with a concentration of 100 μM on the rat anococcygeus muscles did not show any significant change of the CRC of carbachol, whereas (-)-hyoscyamine (0.1 μM) in a single experiment shifted the CRC of carbachol to the right (data not shown). The equilibrium dissociation constant Kβ of (-)-hyoscyamine was calculated as 0.3 nM.
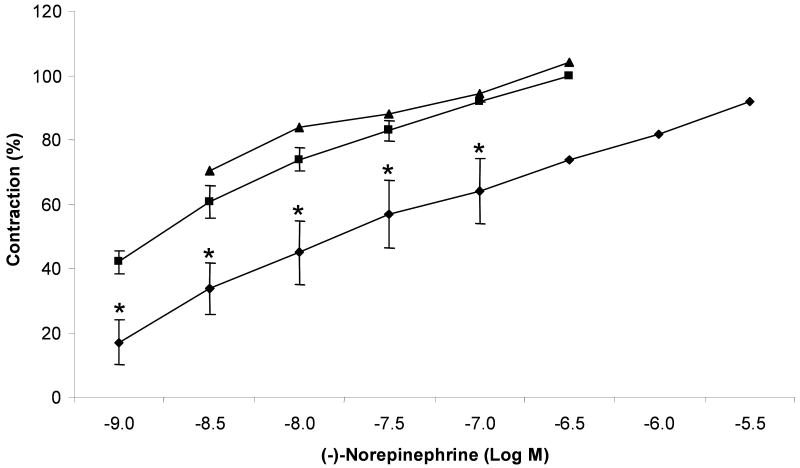
For the rat aorta rings, the effects of (±)-pervilleine A HCl (100 μM) were examined (Figure 2). The maxima of CRC of the α-adrenoreceptor activator, (-)-norepinephrine, which occurred at 0.3 μM in the control observation, was reduced to 74±10% in the presence (30 min) of pervilleine A HCl (100 μM). The blocking action of (±)-pervilleine A HCl seemed to be non-specific or at the level of Ca2+ that is ultimately responsible for the mechanical contraction. In duplicate experiments, (±)-pervilleine H at 100 μM did not block the vascular α-adrenoreceptor-mediated actions of (-)-norepinephrine.
Figure 2.
Concentration response curves to (-)-norepinephrine (n = 2 to 4) obtained in control (■) or in the presence of (±)-pervilleine A HCl (n = 2 to 4) at a concentration of 100 μM (◆) and (±)-pervilleine H (n = 2) at 100 μM (▲). Results were expressed as mean ± SEM of experiments on the rat aorta ring. * p < 0.05, significant difference from the control groups.
As compared to the rat aorta that lacks sympathetic nerves (Patil et al., 1972), the vas deferens is densely innervated by the post-ganglionic sympathetic nerves. Exogenous norepinephrine produces a contraction that is mediated by a post-junctional α-adrenoreceptor. The nerve terminals, however, rapidly transport considerable amounts of (-)-norepinephrine across the neuronal membrane by the uptake1. The well-known tropane alkaloid, (-)-cocaine, blocks the uptake1 of norepinephrine and increases the concentration of the agonist for the greater effect, referred to as the potentiation of norepinephrine response by (-)-cocaine. In this experiment, (-)-norepinephrine at 10 μM produced 90% contraction of the tissue. In the presence of (±)-pervilleine A HCl (100 μM) for 15 min, (-)-norepinephrine produced 90% of the contraction. The same concentration of (±)-pervilleine H did not significantly affect the contraction of (-)-norepinephrine.
In conclusion, these data indicate that (±)-pervilleine A HCl has weak nonspecific cholinergic and vascular adrenergic blocking activities whereas (±)-pervilleine H was found to be inactive in all in vitro test systems used in the present study, in spite of the structural similarity of these tropane alkaloids to (-)-hyoscyamine and (-)-cocaine.
Acknowledgments
Partial funding by the National Cancer Institute, NIH through contract N01-CO-12400, and grant 7U19 CA52956-15, and through the RAID program is gratefully acknowledged.
References
- Brown JH, Taylor P. Muscarinic receptor agonists and antagonists. In: Hardmann JG, Limbird LE, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 10th. McGraw-Hill; New York: 2001. pp. 155–173. [Google Scholar]
- Catterall W, Mackie K. Local anesthetics. In: Hardmann JG, Limbird LE, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 10th. McGraw-Hill; New York: 2001. pp. 367–384. [Google Scholar]
- Chin YW, Jones WP, Waybright TJ, et al. Tropane aromatic ester alkaloids from a large-scale re-collection of Erythroxylum pervillei stem bark obtained in Madagascar. J Nat Prod. 2006;69:414–417. doi: 10.1021/np050366v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinghorn AD, Pezzuto JM, Cui B, Silva GL, You M. Isolation of tropane alkaloid multidrug resistance inhibitors from Erythroxylum pervillei and their use for treatment of cancer and infections. US Patent 6989396 B2. 2006 Jan 24; [Google Scholar]
- Mi Q, Cui B, Chavez H, et al. Characterization of tropane alkaloid aromatic esters that reverse the multidrug resistance phenotype. Anticancer Res. 2002;22:1385–1397. [PubMed] [Google Scholar]
- Mi Q, Cui B, Lantvit D, et al. Pervilleine A, a novel tropane alkaloid that reverses the multidrug-resistance phenotype. Cancer Res. 2001;61:4030–4037. [PubMed] [Google Scholar]
- Patil PN. Pharmacological quantitation. Indian J Exp Biol. 1996;34:615–633. [PubMed] [Google Scholar]
- Patil PN, Fudge K, Jacobowitz D. Steric aspects of adrenergic drugs. 18. α-adrenergic receptors of mammalian aorta. Eur J Pharmacol. 1972;19:79–87. doi: 10.1016/0014-2999(72)90079-9. [DOI] [PubMed] [Google Scholar]
- Silva GL, Cui B, Chavez D, et al. Modulation of the multidrug-resistance phenotype by new tropane alkaloid aromatic esters from Erythroxylum pervillei. J Nat Prod. 2001;64:1514–1520. doi: 10.1021/np010295+. [DOI] [PubMed] [Google Scholar]