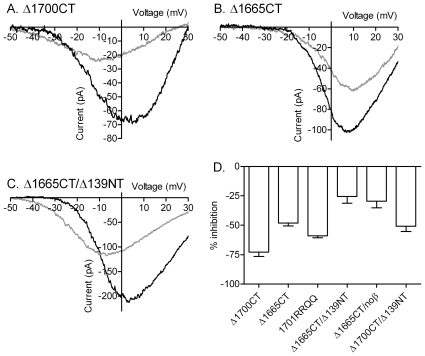
Figure 6. Contributions of the C terminus to anion sensitivity of CaV1.2 currents.
A. Long NT CaV1.2 current after deletion of the C terminus at residue 1700 (Δ1700CT) in control conditions (black trace) and after replacing chloride with gluconate (gray trace). B. CaV1.2 current after deletion of the C terminus at residue 1665 (Δ1665CT) in control (black trace) and gluconate (gray trace) conditions. C. IBa from a mutant lacking both C- and N-termini (Δ1665/Δ139) in control (black trace) and gluconate (gray trace) conditions. D. Bar graph comparing the inhibition of IBa peak amplitude produced by gluconate replacement in the different mutants. Truncating the C terminus of rabbit CaV1.2 at AA 1700 (Δ1700) showed no reduction in gluconate inhibition (−72.3±2.0%, N = 4; ΔGmax −73.9%) compared to full length CaV1.2. However, removing an additional 35 residues by truncating the C terminus at AA 1665 (Δ1665) reduced gluconate inhibition significantly (P<0.0001) to 48.1±2.5% (N = 23; ΔGmax −45.8%). Two positively charged residues in this region were neutralized in a Δ1701 truncation mutant by replacing arginine with glutamine at AA1696 and 1697. Replacing these two residues reduced gluconate inhibition to 58.0±2.1% (N = 7; ΔGmax −43.0%). Inhibition was more strongly reduced in channels lacking both C- and N-termini (Δ1665/Δ139: −25.4±6.9%, N = 5, comparison to Δ1665 with β subunits, P<0.0011; ΔGmax −35.4%) as well as in Δ1665 channels expressed without β subunits (−29.4±5.8%, N = 7, comparison to Δ1665 with β subunits, P<0.003; ΔGmax −25.6%). Gluconate inhibition in the Δ139/Δ1700 double mutant (−50.8±4.4%, N = 7; ΔGmax −41.0%) did not differ from gluconate inhibition in the Δ139 mutant. Except where specified, α1 subunits were co-expressed with α2δ and β2A.