Figure 2.
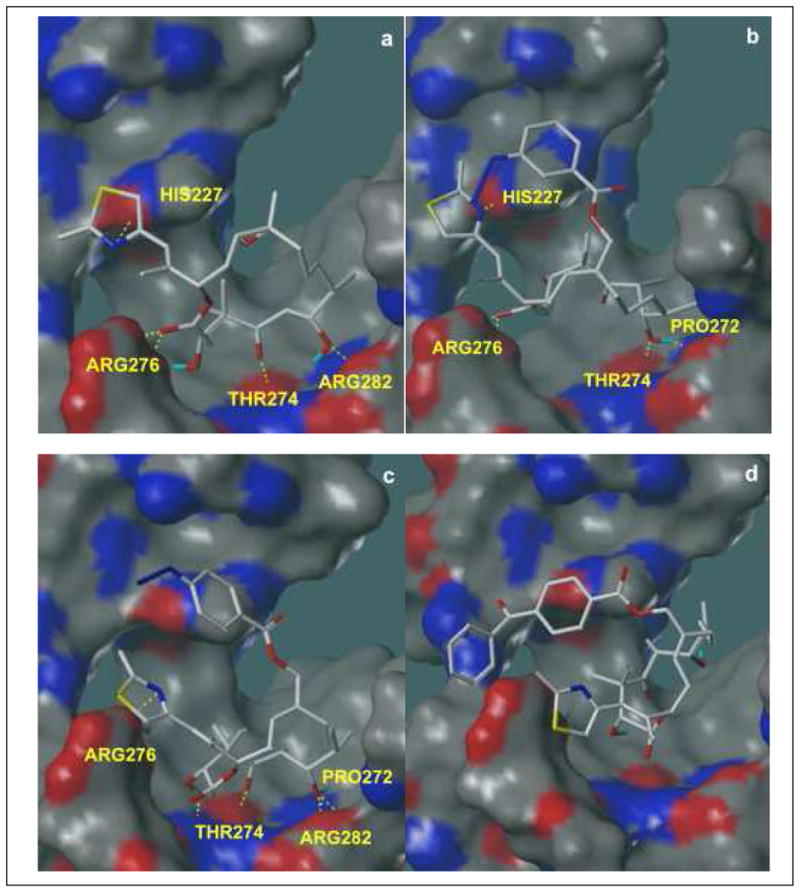
Docked configurations (Surflex-Dock, Tripos, Inc.) of epothilone B (a), compound 14 (b), compound 15 (c) and compound 16 (d) shown with MOLCAD (Tripos, Inc.) electron density surfaces of the tubulin active site (1TVK1), onto which hydrogen bond donor and acceptor regions have been mapped. Red areas represent hydrogen bond donors; blue areas represent hydrogen bond acceptors; and gray indicates regions in which no hydrogen bonding takes place. Key ligand-receptor interactions are shown in these pictures. In the docked conformation of epothilone B (a), hydrogen bonding occurs between the thiazole nitrogen and the imidazole NH of His227; between the C1 carbonyl and two amino groups in Arg276; between the C3 carbonyl and backbone NH of Thr274; and between C5 OH and one amino group in Arg282. In the docked conformation of 14 (b), hydrogen bonding occurs between the thiazole nitrogen and the imidazole NH of His227; between the C1 carbonyl and two amino groups in Arg276; between the C7 OH and backbone carbonyl of Pro272; and between C7 OH and backbone NH of Thr274. In the docked configuration of 15 (c), hydrogen bonding occurs between the thiazole nitrogen and one of the amino groups of Arg276; between the C3 OH and the backbone carbonyl of Thr274; between the C5 carbonyl and backbone NH of Thr274; between the C7 OH and the two amino groups in Arg282; and between the C7 OH and backbone carbonyl of Pro272. Overall, the hydrogen bonding networks between receptor and ligand are preserved in (b) and (c). However, all of these interactions disappear in the docked conformation of 16 (d) due to sterically driven ligand rearrangement. As shown, the molecule rearranges to avoid steric clashes between the receptor and the bulky group on C12.
