Abstract
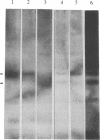
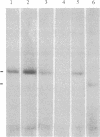
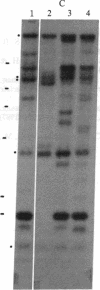
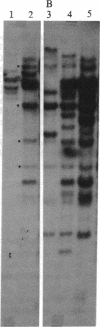
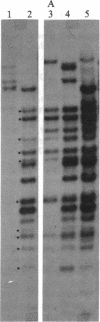
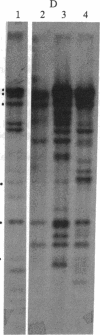
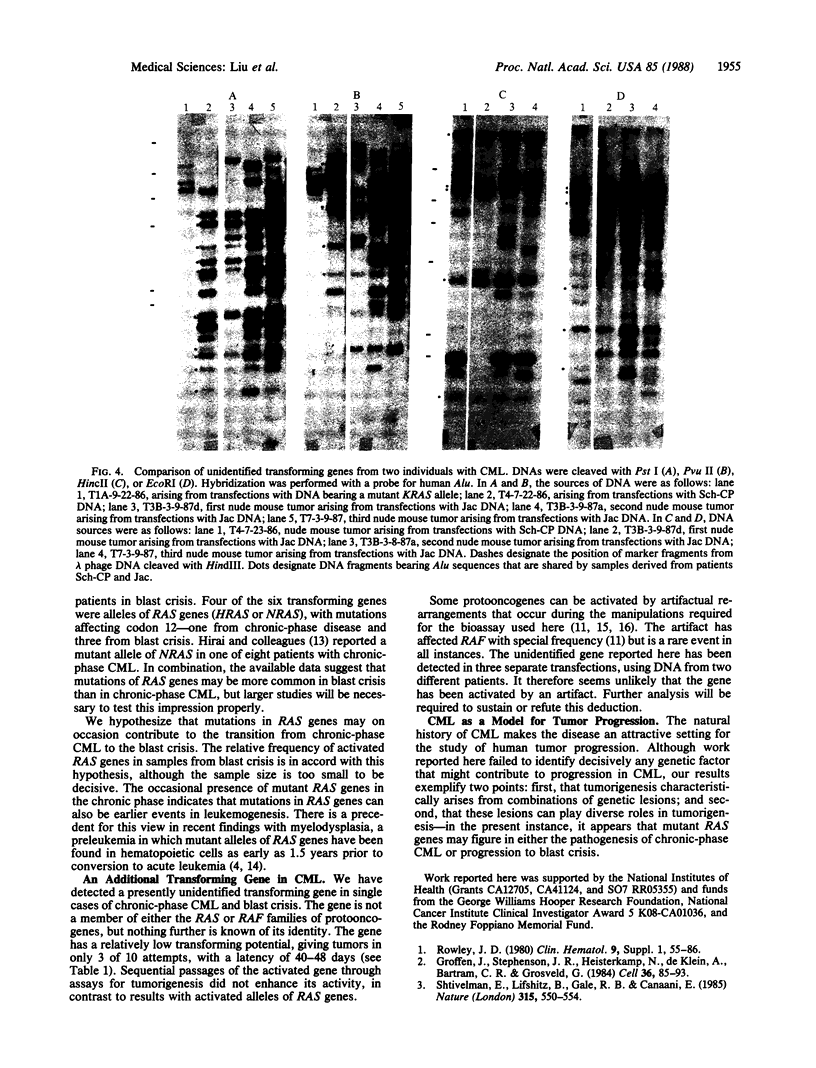
Chronic myelogenous leukemia (CML) is a hematopoietic malignancy characterized by an indolent chronic phase that invariably leads to a "blast crisis" indistinguishable from acute leukemia. Using a sensitive assay based on gene transfer and tumorigenesis, we sought evidence that damage to protooncogenes might figure in the progression from the chronic to the blast phase of CML. Seven of the 12 patients with CML examined in this manner harbored transforming genes. Mutations in RAS protooncogenes were detected in the leukemic cells from 1 of 6 chronic-phase patients, and 3 of 6 blast-crisis patients. In addition, a presently unidentified transforming gene (neither RAS nor RAF) was detected in 1 patient with chronic phase and 1 with blast crisis. Our data indicate that mutations in RAS genes may play diverse roles in the pathogenesis of CML.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Toksoz D., Marshall C. J., Verlaan-de Vries M., Veeneman G. H., van der Eb A. J., van Boom J. H., Janssen J. W., Steenvoorden A. C. Amino-acid substitutions at codon 13 of the N-ras oncogene in human acute myeloid leukaemia. 1985 Jun 27-Jul 3Nature. 315(6022):726–730. doi: 10.1038/315726a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Verlaan-de Vries M., Jansen A. M., Veeneman G. H., van Boom J. H., van der Eb A. J. Three different mutations in codon 61 of the human N-ras gene detected by synthetic oligonucleotide hybridization. Nucleic Acids Res. 1984 Dec 11;12(23):9155–9163. doi: 10.1093/nar/12.23.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L., Verlaan-de Vries M., van der Eb A. J., Janssen J. W., Delwel R., Löwenberg B., Colly L. P. Mutations in N-ras predominate in acute myeloid leukemia. Blood. 1987 Apr;69(4):1237–1241. [PubMed] [Google Scholar]
- Fasano O., Birnbaum D., Edlund L., Fogh J., Wigler M. New human transforming genes detected by a tumorigenicity assay. Mol Cell Biol. 1984 Sep;4(9):1695–1705. doi: 10.1128/mcb.4.9.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B., Droller M. J., Baylin S. B., Nelkin B. D. Mutation affecting the 12th amino acid of the c-Ha-ras oncogene product occurs infrequently in human cancer. Science. 1983 Jun 10;220(4602):1175–1177. doi: 10.1126/science.6304875. [DOI] [PubMed] [Google Scholar]
- Fukui M., Yamamoto T., Kawai S., Mitsunobu F., Toyoshima K. Molecular cloning and characterization of an activated human c-raf-1 gene. Mol Cell Biol. 1987 May;7(5):1776–1781. doi: 10.1128/mcb.7.5.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Hirai H., Kobayashi Y., Mano H., Hagiwara K., Maru Y., Omine M., Mizoguchi H., Nishida J., Takaku F. A point mutation at codon 13 of the N-ras oncogene in myelodysplastic syndrome. Nature. 1987 Jun 4;327(6121):430–432. doi: 10.1038/327430a0. [DOI] [PubMed] [Google Scholar]
- Hirai H., Tanaka S., Azuma M., Anraku Y., Kobayashi Y., Fujisawa M., Okabe T., Urabe A., Takaku F. Transforming genes in human leukemia cells. Blood. 1985 Dec;66(6):1371–1378. [PubMed] [Google Scholar]
- Liu E., Hjelle B., Morgan R., Hecht F., Bishop J. M. Mutations of the Kirsten-ras proto-oncogene in human preleukaemia. Nature. 1987 Nov 12;330(6144):186–188. doi: 10.1038/330186a0. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Ph1-positive leukaemia, including chronic myelogenous leukaemia. Clin Haematol. 1980 Feb;9(1):55–86. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Ritz J., Cooper G. M. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985 Sep;42(2):581–588. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- Young D., Waitches G., Birchmeier C., Fasano O., Wigler M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell. 1986 Jun 6;45(5):711–719. doi: 10.1016/0092-8674(86)90785-3. [DOI] [PubMed] [Google Scholar]