Abstract
Objective
The use of non-platinum drugs in concurrent chemoradiation in carcinoma cervix has not been well explored and hence a two arm study was planned to compare the outcome of concomitant cisplatin or gemcitabine in locally advanced carcinoma cervix.
Methods
Thirty six patients were evaluated in this study for response rates and complications. These patients were divided into two arms, sixteen patients in the cisplatin arm and twenty patients in the gemcitabine arm. Cisplatin and gemcitabine were given as i.v. infusion at doses of 40 mg/m2 and 150 mg/m2 respectively for five weeks concomitant with radiotherapy. All patients had received pelvic radiotherapy to a dose of 50 Gy/25 fraction/5 weeks by four field box technique followed by high-dose-rate brachytherapy (3 sessions, each of 7.5 Gy to point A).
Results
Median follow up was of 10.4 months (range, 3 to 36 months) and 10.9 months (range, 2 to 49 months) in the cisplatin and gemcitabine arms, respectively. At first follow up, 68.8% in the cisplatin arm and 70% in the gemcitabine arm had achieved complete response (p=0.93). Similar response rates were noted in different stages in both arms. None of the patients except one developed grade 4 toxicity. Similar toxicity profiles were observed in both arms. Local disease control, distant disease free survival and overall survival was 68.8% vs. 70%, 93.8% vs. 85%, 68.8% vs. 60% in the cisplatin and gemcitabine arms, respectively.
Conclusion
Weekly gemcitabine had similar disease control and tolerable toxicity profile with cisplatin. Gemcitabine may be used as an alternative to cisplatin in patients with compromised renal function.
Keywords: Chemoradiation, Locally advanced carcinoma cervix, Weekly cisplatin, Gemcitabine
INTRODUCTION
Carcinoma of the uterine cervix is the most common malignancy amongst females in India. Early invasive cervical cancer can be successfully treated with radical surgery or radiotherapy alone, or by combined approach. Radiation treatment has been the standard definitive therapy for patients with large cervical cancers confined to cervix, and for patients with locally advanced cancers until the beginning of ninth decade. Loco-regional failure when treated with radiotherapy alone is significant: 25 to 30 percent for IIB and 30 to 40 percent for stage III-IVA. To improve the therapeutic ratio, chemotherapy was introduced in the treatment of carcinoma cervix, either as a single agent or in combination as neoadjuvant, adjuvant or concurrent protocols. In the setting of concurrent chemoradiotherapy the famous five trials were published with enthusiastic results, and subsequently in February 1999, U.S. National Cancer Institute (NCI) stated that, "Concurrent chemotherapy should be incorporated in women who require radiation therapy for treatment of cervical cancer."1
Since then, concurrent chemo-radiation became the accepted standard of care for carcinoma of the cervix. In 2005, the Cochrane database systemic review2 of concurrent chemoradiation in carcinoma of the cervix also reiterated an absolute benefit of 10% in overall survival and 13% in progression free survival regardless of whether or not platinum was used.
Hence, amongst many chemotherapeutic options, the ideal drug(s), their combination and schedule is still a matter of exploration. Although most of the trials showed cisplatin is the most efficacious, the jury is still out whether cisplatin is the best drug available in the concurrent setting.
To improve the survival rates and tolerability, there is a need to explore the use of alternative chemotherapeutic agents. A variety of agents such as carboplatin, paclitaxel, 5-fluorouracil (5-FU) have been studied with good results in cervical cancer.2
Gemcitabine which is a deoxycytidine analog, is a radiosensitizer at low doses and also shows synergistic effects with cisplatin.3 Gemcitabine has been used in cervical cancers with good results both as a single agent and in combination with cisplatin concurrent with radiotherapy.4-14 The aim of present study was to evaluate the toxicity and efficacy of concurrent weekly Gemcitabine against the widely accepted concurrent weekly cisplatin with standard convention radiotherapy in management of locally advanced cervical cancer.
MATERIALS AND METHODS
1. Patient characteristics
A total of 36 patients of newly diagnosed cases of cervical malignancy were enrolled in this study from July 2004 to June 2005. All patients were previously untreated. Staging was performed according to the International Federation of Gynaecology and Obstetrics (FIGO) cancer staging system. Staging was cross checked by independent examiners. A thorough clinical examination was performed including per-speculum examination, per-vaginal examination, digital rectal examination, and per-abdominal examination, In all patients, investigations such as Chest X-ray, Ultrasonography abdomen or contrast enhanced computed tomography (abdomen and pelvis), cystoscopy and sigmoidoscopy were carried out. Para-aortic lymph nodal mapping was not done.
Patients were required to have 1) squamous histopathogy, 2) FIGO stage II and III, 3) Karnofsky performance status of ≥ 70, 4) adequate hematological functions with haemoglobin (Hb) level >10 g/dl (whole blood transfusion was done to achieve this level in anaemic patients), total leukocyte counts >4,000 cells/µl, platelets count >100×103/µl, 5) normal renal function with blood urea <40 mg/dl, serum creatinine <1.5 mg/dl, 6) adequate liver function serum glutamic-oxaloacetic transaminase <35 IU/L, serum glutamic pyruvic transaminase <35 IU/L, and 7) no prior chemotherapy or radiotherapy.
Criteria for exclusion were the presence of fistulas, stage IV disease (metastasis in distant organs such as the liver, lung, and bones etc), patients with malignant ascites, and previously treated cases of cases of carcinoma of the cervix by radiotherapy or chemotherapy.
2. Radiotherapy
The conventional pelvic radiotherapy was planned in Co-60 unit to the whole pelvis by parallel apposed anterior-posterior portals or four field box technique. Midline shield was not done. Delivered dose was 50 Gy in 5 weeks / 5 fractions per week. It was followed 1 to 2 weeks later by 3 sessions of high-dose-rate (HDR)-brachytherapy. Upper, lateral and lower borders of the anterior and posterior pelvic fields were at L4-L5 inter-vertebral space, 1.5 to 2 cm lateral to the widest bony pelvic wall and inferior border of obturator foramen. In cases with vaginal extension, the inferior border was kept at the vaginal introitus. For lateral portals, the anterior border was at the pubic symphysis and the posterior border at the S2-S3 inter-vertebral space. High dose rate brachytherapy was delivered by Fletcher & Suit's tandem and ovoid application and delivered dose to point A was 7.5 Gy in three sessions each.
The doses used (50 Gy external beam radiotherapy+7.5 Gy ×3 sessions HDR brachytherapy) would result in Point A dose of BED 10 of 82.7 Gy or BED 3 of 97.4 Gy.
Radiotherapy was delivered within 4 to 6 hours of chemotherapy administration.
3. Chemotherapy
Concurrent chemotherapy commenced within the first 3 days of starting of radiotherapy. Patients in the cisplatin arm received conventional radiotherapy with concurrent cisplatin 40 mg/m2 weekly as intravenous infusion. Pre-medication with Ondansetron 8 mg intravenous push and hydration with 1 liter of intravenous fluid was given before cisplatin infusion. Cisplatin was diluted with normal saline and infused at a rate of 1 mg/minute. Following cisplatin infusion, 300 ml of 20% mannitol and one liter of intravenous fluid with 1 gm of magnesium sulfate was infused, and patients were advised to take oral anti-emetics for 3 days.
Patients in the gemcitabine arm received conventional radiotherapy with concurrent gemcitabine 150 mg/m2 weekly as intravenous infusion. Pre-medication with ranitidine 50 mg, dexamethasone 8 mg, ondansetron 8 mg intravenous push was given before gemcitabine infusion. Gemcitabine was diluted in 150 ml of normal saline and infused over 30 minutes. No pre- or post-hydration was given with gemcitabine infusion.
Chemotherapy was withheld in patients who developed grade 3 lower gastrointestinal toxicity, leukocyte count < 2,000 cell/µl, thrombocytopenia <100×103/µl, and patients with rising liver function tests. Patients were managed conservatively with intravenous fluid supplementation and prophylactic oral antibiotics. Chemotherapy was restarted in patients whose toxicity regressed and achieved normal leukocyte and platelet counts, while it was cancelled in patients with persistent toxicity.
4. Response and toxicity evaluation
Acute and late toxicity was assessed by the Radiation therapy Oncology Group criteria. All patients were examined weekly for toxicity evaluation in the form of skin reaction, upper and lower gastrointestinal toxicity, hematological toxicity, renal toxicity and weight loss.
Responses were evaluated after one month of completion of treatment. The complete regression of all clinically detectable disease was designated as complete response (CR), and marked symptomatic improvement with 50% or greater regression as partial response. No response (NR) was defined as no significant change in disease, while appearance of new lesions or increase of clinically detectable disease was labelled as progressive disease (PD).
Follow up of patients were done every 3 months for the initial two years, 6 monthly for the 3rd to 5th year, and thereafter annually. Clinical history, pelvic, abdominal and nodal examination was performed during each follow up. Radiological and cyto-pathological investigation was done only when indicated.
5. Statistical analysis
Analysis was done using SPSS (SPSS Inc., Chicago, IL, US). Chi-square and ANOVA test were used to compare patient characteristics, toxicity and response. Toxicity profile was grouped into low grade (grade 0, 1, 3) and high grade (grade 3 and 4), and compared with Fisher's exact test. Disease free survival (DFS) was analyzed from the date of registration, local or distant relapse, death or until the last visit. Curves were plotted using the Kaplan-Meier method and compared with the log rank test.
RESULTS
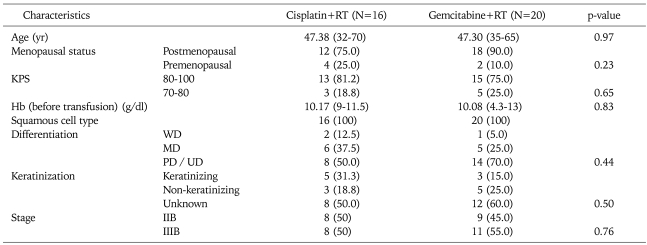
Out of 36 patients enrolled in the study, 16 patients were included in the cisplatin arm as the control group, while 20 patients were in the gemcitabine arm as the study group. The study and control group characteristics are shown in Table 1 and were seen to be well matched.
Table 1.
Patient characteristics
Values are presented as number (%) or median (range).
RT: radiotherapy, KPS: Karnofsky performance status, Hb: haemoglobin, WD: well differentiated, MD: moderately differentiated, PD: poorly differentiated, UD: undifferentiated /unknown.
1. Response evaluation
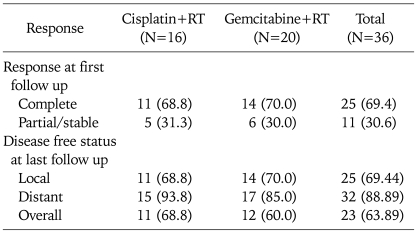
Out of 36 patients, 25 (69.4%) had achieved complete loco-regional control at one month of completion of treatment. A similar result in complete response was observed in both cisplatin and gemcitabine arms i.e. 68.8% and 70% respectively (p=0.93) as shown in Table 2. The disease status at last follow up is also shown in Table 2.
Table 2.
Response after one month from the completion of treatment
Values are presented as number (%).
RT: radiotherapy, NED: no evidence of disease.
It was seen that complete response at first follow up after 1 month of completion of treatment was a significant factor influencing disease control on further follow up. Median duration of follow up was similar 10.4 months (range, 3 to 36 months) and 10.9 months (range, 2 to 43 months) months in cisplatin and gemcitabine arm (p=0.88). It was seen that a higher number of patients achieved overall and distant disease free status in the cisplatin arm than the gemcitabine arm i.e. 68.8% vs. 60% (p=0.58) and 93.8% vs. 85% (p=0.40). Similarly, local control was comparable in both arms (68.8% vs. 70%) (p=0.93). However, these differences were not statistically significant.
Patients with complete response were less likely to experience treatment failure at a later stage. There was no relapse among complete responders in the cisplatin arm, while three such cases in the gemcitabine arm developed either local or distant failure after achieving complete response. While one patient relapsed at both local and distant sites, two patients of the gemcitabine arm developed only distant metastasis. These observations are at a limited follow up and may not be sustainable at longer follow up.
Our study has severe limitations due to limited number of patients and sizable loss of follow up of patients. As the majority of the patients belonged to poor socio-economic status, after completion of treatment their long term follow up was far from satisfactory.
2. Toxicity
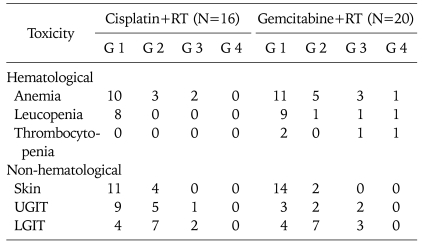
Similar toxicity profiles were observed in both arms (Table 3). Compared to the cisplatin arm, a similar number of patients in the gemcitabine arm developed grade 3 and 4 anemia (4/20 vs. 2/16; p=0.67), leukopenia (2/20 vs. 0/16; p=0.49) and thrombocytopenia (2/20 vs. 0/16; p=0.49). Apart from one patient in the gemcitabine arm who developed grade 4 neutropenic sepsis and had pancytopenia that required platelet and blood transfusion, none of the other patients developed grade 4 toxicity. Grade 1/2 upper gastrointestinal toxicity was more common in the cisplatin arm as compared to the gemcitabine arm (14/16 vs. 5/20; p=1.00). The skin reactions and lower gastrointestinal toxicity were similar in both arms (p=1.00).
Table 3.
Acute toxicity (RTOG criteria)
RTOG: The Radiation Therapy Oncology Group, G: grade, UGIT: upper gastrointestinal toxicity, LGIT: lower gastrointestinal toxicity.
Three patients developed late reaction in the form of subcutaneous fibrosis, vaginal stenosis and rectal complications in form of rectal bleeding, subacute intestinal obstruction at last follow up. None of the patients developed any late urinary bladder complications.
DISCUSSION
Carcinoma of the cervix remains the biggest challenge to treat in a developing country like India. Most of the cases present in advanced stages. After the NCI alert in 1999, chemoradiation with cisplatin was accepted as a new standard of care. Cisplatin weekly is well tolerated and shows better results as compared to radiotherapy alone in studies from the Indian subcontinent as well. However cure rates remain low and reported best 5 years survival rates only touches 60%.
Non platinum compounds alone have been infrequently analyzed in concurrent chemoradiation protocols and have been generally ignored in the literature. 5-FU along with cisplatin was tried in a few trials but the toxicities were the issue, and also the aspect of frequent treatment breaks was emphasized. On the basis of five trials on which cisplatin became the standard of care, four of them had 5-FU in combination.1 Similarly, gemcitabine remains another underutilized drug in the concurrent chemoradiation setting in carcinoma of the cervix. Due to lack of studies, we do not have much data on its efficacy compared to the standard regimen of concurrent cisplatin in carcinoma of the cervix.
Gemcitabine belongs to the antimetabolite group (2', 2'-difluorodeoxycytidine) that inhibits DNA synthesis, eventually causing apoptosis. Gemcitabine has shown efficacy against a variety of solid tumors, most notably against pancreatic and lung cancer. It has been also investigated in breast, gastrointestinal (GI) malignancies, ovary, urinary bladder and cervix cancer.3 Gemcitabine was initially tried in metastatic and recurrent cervical cancers showing moderate activity. The results have been relatively modest when given alone in recurrence or post radiation residual disease.4-7 Due to less than expected results, gemcitabine is rarely employed alone to treat carcinoma of the cervix in a recurrence/residual setting.
From in vitro and in vivo studies it was realized that gemcitabine is a potent radiosensitizer, which when combined with radiotherapy improves the results dramatically. It also shows synergistic action with cisplatin, the probable mechanism being DNA-cisplatin adduct's repair inhibition by gemcitabine.3
Pattaranutaporn et al.11 in a phase II study has shown that when gemcitabine alone combined with pelvic radiation in stage IIIB patients resulted in excellent pelvic control rate with 84.2% disease-free survival and 100% overall survival at a median follow-up of 19.9 months.
Porras et al.12 treated 24 patients with locally advanced disease with a fixed dose of gemcitabine (300 mg/m2 weekly) and concurrent radiotherapy. Toxicity was mild and a complete response rate of 89% was reported.
In the present study, complete response was observed in 70% of cases and 20% showed partial response, while one case did not show any response in the gemcitabine arm.
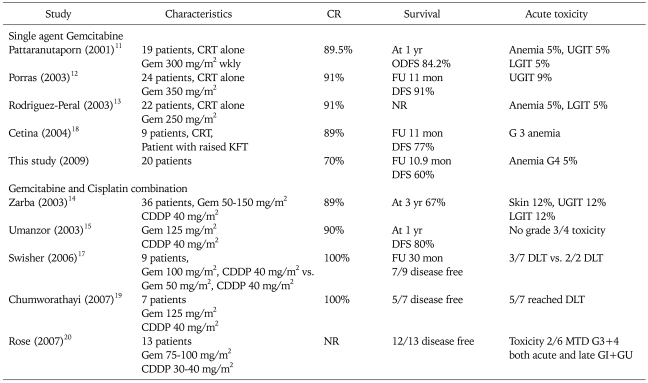
Complete response rates seen were inferior to that achieved by Pattaranutaporn et al.11 (89.5%), Porras et al.12 (91.6%), and Rodriguez-Peral13 (91%). The cause of our lower response rates compared to other studies may be high doses of gemcitabine (350 mg/m2 in Porras study12 and 250 mg/m2 Rodriguez-Peral study13) and addition of lower stage disease IB2 and more number of cases with stage IIA. This also explains the slightly higher disease free survival at 1 year, i.e. 84% by Pataranutaporn et al.11 and 91% by Porras et al.12 at 9 months. Pelvic and distant failure corresponds to as reported by Pataranutaporn et al.11 (11% and 5%) and Rodriguez-Peral13 (9% pelvic failure). Toxicity profiles were similar to other studies (Table 4). In our experience, gemcitabine was easy to administer, well tolerated with good compliance to concurrent chemoradiation.
Table 4.
Concomitant Gemcitabine with radiotherapy
CR: complete response, CRT: concomitant chemo-radiotherapy, Gem: gemcitabine, ODFS: overall disease free survival, UGIT: upper gastrointestinal toxicity, LGIT: lower gastrointestinal toxicity, CDDP: cis-platinum, FU: median follow-up, KFT: kidney function test, G: grade, MTD: maximum tolerable dose, DLT: dose limiting toxicity, GI: gastro-intestinal, GU: gastro-urinary, NR: not reported.
With a view to exploit the synergy of cisplatin and gemcitabine, this doublet is being tried in concurrent chemoradiation trials. Zarba et al.14 conducted phase I-II study in stages IIB-IVA disease and administered cisplatin at 40 mg/m2 along with escalating doses of gemcitabine. The results showed disease free survival in 78% of patients at a median follow-up of 12 months. Another equally significant study is from Umanzor et al.15 whose treatment consisted of: cisplatin 40 mg/m2, followed by gemcitabine 125 mg/m2, once weekly, given 1 to 2 hours before radiotherapy, and 20/23 patients were evaluable for response. The complete response rate seen was 90% (18/20), and the partial response rate was 10% (2 patients with persistent disease after therapy). The toxicities were mild to moderate. At a median follow-up of 12 months, all patients were alive, and 16/20 (80%) were disease-free.
In the study from the Indian subcontinent by Bhatt et al.16 phase III trial was conducted. They compared cisplatin 35 mg/m2 weekly with gemcitabine 150 mg/m2 weekly, along with radiation therapy. They analyzed sixty patients with 32 patients in the cisplatin (control arm) and 28 in the gemcitabine (study arm). The reported complete response rate was 89% in the gemcitabine arm vs. 72% in the control arm (p<0.05). However, overall response rate (CR+PR) was similar in both groups (96% in study group vs. 100% in control group). Grade III GI toxicity was higher in the study group as compared to controls (14% vs. 3%), and one patient had Grade III skin reaction. Hence in this study it was seen that gemcitabine had a higher rate of complete responses, even though the authors chose the dose of cisplatin at 35 mg/m2, which is slightly lower than the standard accepted dose of 40 mg/m2. Since the tolerance of the regimen remains a concern, the lowering of cisplatin weekly doses seems to be an option to be further explored. In interesting observations by Swisher et al.17 when gemcitabine is administered before cisplatin, the toxicities are higher. According to their study, gemcitabine even at low doses of 50 mg/m2 is the maximum tolerable dose if combined with cisplatin 40 mg/m2 weekly. But the response rates were very high and the authors advocate further exploration. The rest of the studies in concurrent chemoradiation with both gemcitabine and cisplatin have utilized sequencing of cisplatin first followed by gemcitabine, and have shown more tolerability. Higher doses of gemcitabine could be administered along with cisplatin to the range of 150 mg/m2 to 200 mg/m2 without the need for lowering cisplatin doses from the standard dose of 40 mg/m2.
Cetina et al.18 administered weekly gemcitabine in seven patients with renal dysfunction and reported normalization of renal function with excellent disease control. Hence in gemcitabine, we have a viable option of providing the benefit of concurrent chemoradiation in cases of carcinoma of the cervix with renal dysfunction where cisplatin is contraindicated.
Weekly gemcitabine had similar disease control and tolerable toxicity profile with cisplatin. Gemcitabine can be a viable option as alternative to cisplatin in selected cases in which cisplatin is contraindicated due to renal compromise.
Footnotes
Abstract of this paper accepted as poster presentation at ECCO 15/ESMO 34 - Berlin 20-24 September 2009.
References
- 1.Thomas GM. Concurrent chemotherapy and radiation for locally advanced cervical cancer: the new standard of care. Semin Radiat Oncol. 2000;10:44–50. doi: 10.1016/s1053-4296(00)80020-x. [DOI] [PubMed] [Google Scholar]
- 2.Green J, Kirwan J, Tierney J, Vale C, Symonds P, Fresco L, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2005;(3) doi: 10.1002/14651858.CD002225.pub2. CD002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Moorsel CJ, Veerman G, Bergman AM, Guechev A, Vermorken JB, Postmus PE, et al. Combination chemotherapy studies with gemcitabine. Semin Oncol. 1997;24(2 Suppl 7):S7-17–S7-23. [PubMed] [Google Scholar]
- 4.Schilder RJ, Blessing J, Cohn DE. Evaluation of gemcitabine in previously treated patients with non-squamous cell carcinoma of the cervix: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2005;96:103–107. doi: 10.1016/j.ygyno.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 5.Burnett AF, Roman LD, Garcia AA, Muderspach LI, Brader KR, Morrow CP. A phase II study of gemcitabine and cisplatin in patients with advanced, persistent, or recurrent squamous cell carcinoma of the cervix. Gynecol Oncol. 2000;76:63–66. doi: 10.1006/gyno.1999.5657. [DOI] [PubMed] [Google Scholar]
- 6.Brewer CA, Blessing JA, Nagourney RA, McMeekin DS, Lele S, Zweizig SL. Cisplatin plus gemcitabine in previously treated squamous cell carcinoma of the cervix: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2006;100:385–388. doi: 10.1016/j.ygyno.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Matulonis UA, Campos S, Duska L, Krasner CN, Atkinson T, Penson RT, et al. Phase I/II dose finding study of combination cisplatin and gemcitabine in patients with recurrent cervix cancer. Gynecol Oncol. 2006;103:160–164. doi: 10.1016/j.ygyno.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Lorvidhaya V, Chitapanarux I, Sangruchi S, Lertsanguansinchai P, Kongdanarat Y, Tangkaratt S, et al. Concurrent mitomycin C, 5-fluorouracil, and radiotherapy in the treatment of locally advanced carcinoma of cervix: a randomized trial. Int J Radiat Oncol Biol Phys. 2003;55:1226–1232. doi: 10.1016/s0360-3016(02)04405-x. [DOI] [PubMed] [Google Scholar]
- 9.Duenas-Gonzalez A, Lopez-Graniel C, Gonzalez A, Gomez E, Rivera L, Mohar A, et al. Induction chemotherapy with gemcitabine and oxaliplatin for locally advanced cervical carcinoma. Am J Clin Oncol. 2003;26:22–25. doi: 10.1097/00000421-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Termrungruanglert W, Tresukosol D, Vasuratna A, Sittisomwong T, Lertkhachonsuk R, Worasethsin P, et al. Neoadjuvant gemcitabine and cisplatin in (bulky) squamous cell carcinoma of the cervix stage IB2 and IIA. ASCO Annual Meeting; 2002. Abstract 2542. [DOI] [PubMed] [Google Scholar]
- 11.Pattaranutaporn P, Thirapakawong C, Chansilpa Y, Therasakvichya S, leumwananontachai N, Thephamongkhol K. Phase II study of concurrent gemcitabine and radiotherapy in locally advanced stage IIIB cervical carcinoma. Gynecol Oncol. 2001;81:404–407. doi: 10.1006/gyno.2001.6197. [DOI] [PubMed] [Google Scholar]
- 12.Porras AR, Valencia N, Bastarrachea J. Weekly gemcitabine concurrently to external radiotherapy for cervical cancer stage IB2-IVA. ASCO Annual Meeting; 2003. Abstract 1987. [Google Scholar]
- 13.Rodriguez-Peral JJ. Locally advanced cervical carcinoma (LACC) treated with concurrent gemcitabine (GEM) and radiotherapy (RT). ASCO Annual Meeting; 2003. Abstract 1988. [Google Scholar]
- 14.Zarba JJ, Jaremtchuk AV, Gonzalez Jazey P, Keropian M, Castagnino R, Mina C, et al. A phase I-II study of weekly cisplatin and gemcitabine with concurrent radiotherapy in locally advanced cervix carcinoma. Ann Oncol. 2003;14:1285–1290. doi: 10.1093/annonc/mdg345. [DOI] [PubMed] [Google Scholar]
- 15.Umanzor J, Aguiluz M, Pineda C, Andrade S, Erazo M. Concurrent Chemoradiation in locally advanced cervical carcinoma (LACC): role of a combination of cisplatin, gemcitabine and radiotherapy- a phase II trial. ASCO Annual Meeting; 2003. Abstract 1953. [Google Scholar]
- 16.Bhatt ML, Matin A, Srivastava M, Pant MC, Srivastava K, Gupta S, et al. Evaluation of gemcitabine versus cisplatinum as an adjunct to radiotherapy in locally advanced carcinoma uterine cervix. ASCO Annual Meeting; 2007. Abstract 16012. [Google Scholar]
- 17.Swisher EM, Swensen RE, Greer B, Tamimi H, Goff BA, Garcia R, et al. Weekly gemcitabine and cisplatin in combination with pelvic radiation in the primary therapy of cervical cancer: a phase I trial of the Puget Sound Oncology Consortium. Gyne Oncol Oncol. 2006;101:429–435. doi: 10.1016/j.ygyno.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Cetina L, Rivera L, Candelaria M, de la Garza J, Duenas-Gonzalez A. Chemoradiation with gemcitabine for cervical cancer in patients with renal failure. Anticancer Drugs. 2004;15:761–766. doi: 10.1097/00001813-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Chumworathayi B, Yuenyao P, Tangvorapongchai V, Luanratanakorn S, Pattamadilok J, Krusun S. Weekly gemcitabine and cisplatin concurrent with pelvic irradiation for primary therapy of cervical cancer: report of the first seven cases in Thai women. Radiat Med. 2007;25:474–479. doi: 10.1007/s11604-007-0171-1. [DOI] [PubMed] [Google Scholar]
- 20.Rose PG, Degeest K, McMeekin S, Fusco N. A phase I study of gemcitabine followed by cisplatin concurrent with whole pelvic radiation therapy in locally advanced cervical cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2007;107:274–279. doi: 10.1016/j.ygyno.2007.06.012. [DOI] [PubMed] [Google Scholar]