Abstract
Context.
The use of thiazolidinediones (TZDs) in patients with type-2 diabetes mellitus appears to be associated with an increased risk of myocardial infarction (MI) compared with placebo or other oral antidiabetic drug regimens.
Objective.
We conducted a study to investigate whether there was a difference in the risk of acute MI and hemorrhagic and non-hemorrhagic stroke between specific TZDs, namely rosiglitazone maleate (Avandia) and pioglitazone (Actos), and other oral antidiabetic agents in a high-risk, largely underrepresented and largely minority Medicaid population.
Study Design, Setting, and Patients.
We analyzed patient encounter data using propensity-scoring methods and logistic regression to compare the risk of cardiovascular (CV) events in patients with type-2 diabetes in a high-risk population.
Main Outcome Measures.
Outcomes were identified through International Classification of Disease (ICD-9) codes 410–411 for acute MI; 430–438 for stroke; and revenue (emergency department) codes 450–459 in the case of MI.
Results.
Using retrospective medical encounter and prescription data analyses, we found that rosiglitazone, compared with other oral antidiabetic agents, was associated with an increased rate of CV events by 20% in a high-risk cohort of diabetic patients. Neither pioglitazone nor the TZD drug class as a whole was associated with an increased CV risk.
Conclusion.
Rosiglitazone was associated with a significant increase in CV events (MI and stroke) among high-risk patients with type-2 diabetes, whereas pioglitazone was not. We recommend further research to capture risk factors that were not observed in our encounter data.
INTRODUCTION
Recent studies have suggested that the use of thiazolidinediones (TZDs) for patients with type-2 diabetes is associated with an increase in the risk of myocardial infarction (MI) and a potential increase in the risk of death from cardiovascular (CV) events.1,2 Approximately two-thirds (65%) of the deaths among patients with diabetes are attributable to heart disease or stroke. For example, it has been observed that patients with end-stage renal disease have CV mortality rates that are 10 to 30 times greater than those of the general population.3
Such findings have raised the question of a possible drug class effect. Our study compared the effects of TZDs with those of other oral antidiabetic drugs (OADs) on CV events in a population of high-risk, primarily minority Medicaid beneficiaries in Maryland.
Two TZDs are currently available: rosiglitazone (Avandia, GlaxoSmithKline) and pioglitazone (Actos, Takeda/Lilly). Combinations of these TZDs and other OADs, such as metformin (Glucophage, Bristol-Myers Squibb) and sulfonylureas, are also available to treat patients with type-2 diabetes. Another TZD, approved in the U.S. in the late 1990s, troglitazone (Rezulin, Sankyo/Parke-Davis), was withdrawn in March 2000 because of its strong association with hepatotoxicity.4
We used propensity scoring and logistic regression models to compare rates of CV events in diabetic patients who were receiving TZDs or OADs. We sought to determine the difference in the odds of acute MI and stroke (hemorrhagic and non-hemorrhagic) associated with the use of TZDs or other OADs, in real-world practice, in a high-risk group that had been largely underrepresented in clinical trials and in other prospective studies.
METHODS
Study Design and Methodology
Our study used de-identified Maryland Medicaid medical encounter and prescription data from all managed care organizations in the state. We analyzed all of these data and the patient’s documented treatment and medication history between January 1, 2001, and June 30, 2006. To reduce the likelihood of CV event prevalence bias, we excluded patients who had submitted their first TZD or OAD claim during the first three months of the study, and we used this time frame as a run-in period.
Nissen and Wolski stated that drug “therapy might provoke MI or death from CV causes after a relatively short-term exposure” if patients belonged to a susceptible population.1 Singh et al. provided similar evidence, stating that “heart failure was more likely to occur after several months (with a median treatment duration of 24 weeks) after initiation of therapy.”5
Our main focus was to determine whether there was a difference in the rate of CV events associated with rosiglitazone and pioglitazone in a head-to-head study involving patients with type-2 diabetes. However, we also assessed whether TZDs as a whole (the drug class effect) increased this risk. We constructed logistic regression models to estimate the probability of an adverse CV event based on TZD or OAD use.
Propensity scoring is particularly useful for controlling treatment effects, or channeling bias, with observational data. This method corrects for the possibility of confounding by indication based on prescribing decisions that take into account a given patient’s baseline risk or other specific morbidities. If the two comparison groups do not share similar characteristics of covariates, the comparison of the treatment effects of the two groups may be overestimated or underestimated. Using this method of scoring helps to control such confounding by maximizing the chance that baseline variables are randomly distributed between the two drug groups within each stratum of the propensity score.
Rosenbaum and Rubin developed this analytical method.6–8 By estimating the probability of exposure to a certain treatment, given multiple covariates, we adjusted the initial model. We used the two-stage model, first predicting the likelihood of TZD treatment (the propensity score) and then used logistic regression to predict the likelihood of events.
When we constructed the propensity score, it was crucial that we adjust the patient samples in order to balance the properties of the covariates. We compared the covariate means for treated patients and control groups, before and after matching, and tested the covariate balance. This step was performed to test whether the mean covariates of patients in one treatment group would differ significantly from those in the other treatment group within a similar propensity score.
We divided the final sample into five quintiles and used a weighted average over the strata in each treatment group to calculate the t-statistic in the groups. We repeated the procedure for each stratum and each covariate until all patient characteristics were in balance.
Data Source and Sample
We initially included a total of 19 million medical records from a total of 20,756 patients, with nine million pharmacy claims, in our sample. More than 14,000 patients with type-2 diabetes mellitus initially were prescribed a TZD or another OAD during the study period. Most patients were female (67%) and African-American (58%), and 39% lived in the city of Baltimore. Only patients who had both medical and pharmacy claims during the study period were included in the study. Among diabetic patients, those who were treated with insulin alone during the entire study period were excluded.
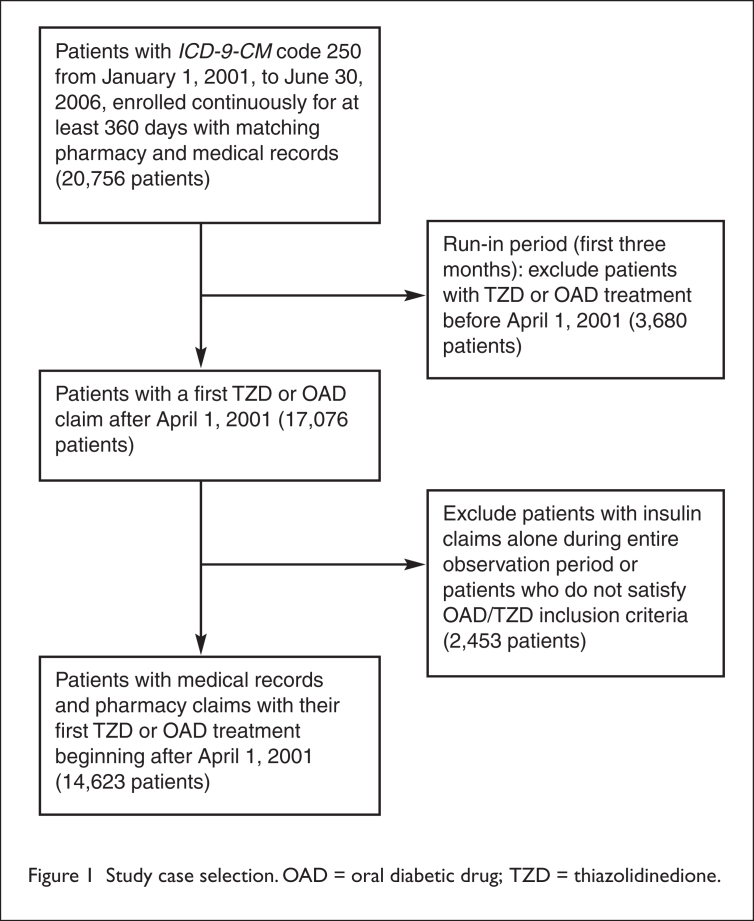
These insulin-treated patients represented one of two groups. The first group had type-2 diabetes at a progressive stage, and the second group had type-1 diabetes mellitus. A flow chart depicting our study case selection is presented in Figure 1.
Figure 1.
Study case selection. OAD = oral diabetic drug; TZD = thiazolidinedione.
Low-income families; children; pregnant women; women with breast or cervical cancer; and aged, blind, or disabled adults sometimes qualify for Medicaid programs. We excluded patients who were dually eligible for both Medicaid and Medicare, because by definition their Medicaid files would be only partially complete.
We controlled for the demographic variables of age (as of January 1, 2007), race, city residency, and sex. The age categories included patients younger than 40 years and those between 40 and 65. A race variable was used to compare African-Americans (51%) with all other patients, because other groups were not represented in our sample. Residents of Baltimore were compared with others in the state. The resultant reference group was the non–African-American male with type-2 diabetes, younger than 40 years of age, and a non-Baltimore resident with no other recorded comorbidity conditions.
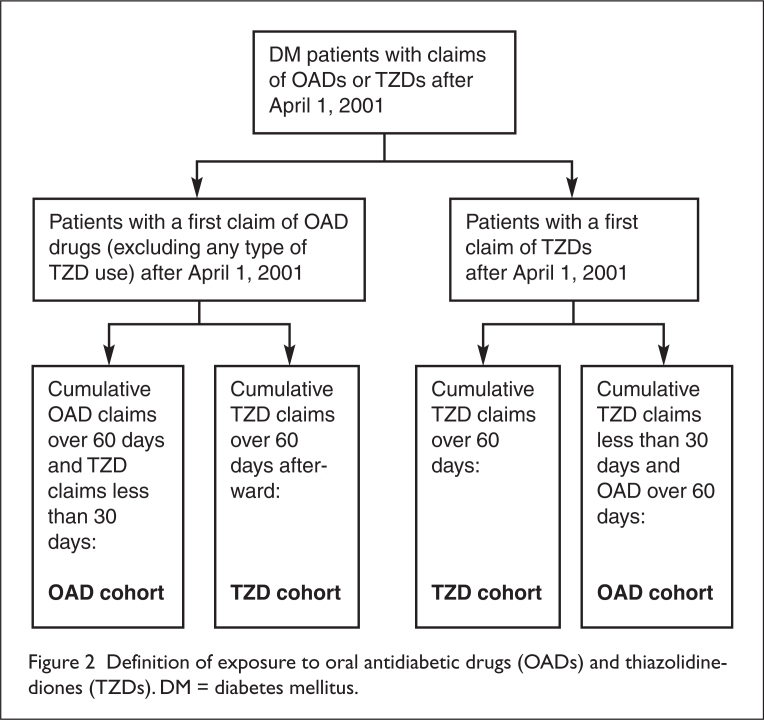
The mean age of the total study population (14,623 patients) was 51 years (median, 53 years). In our final sample, approximately 39% (5,712 patients) who took a certain type of OAD also took the TZDs over 60 cumulative days during the study period. We started observing patients on the first day of receiving their prescription for a TZD or an OAD. We defined “exposure” to a given drug as a cumulative 60 days of taking that drug with no more than 30 days of taking the other drug.9 OAD and TZD cohort definitions are detailed in Figure 2.
Figure 2.
Definition of exposure to oral antidiabetic drugs (OADs) and thiazolidine-diones (TZDs). DM = diabetes mellitus.
We used generic drug codes to identify the information about medication use.10 To investigate the effects of drug doses, we created a new variable (a proxy for dose)—the quantity supply divided by the day’s supply.
We obtained information about the patient’s disease from the first three digits of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes in the medical encounters for diabetes (250) as the primary, secondary, and tertiary diagnoses. Covariates for CV risk factors include hypertension; hyperlipidemia; heart conditions; obesity; and alcohol, tobacco, or drug or substance abuse. We also identified these from the first three digits of ICD-9-CM codes: hypertension (401–404); heart disease (393–398); obesity (278); tobacco, alcohol, or drug or substance abuse (291, 303, and 305); and hyperlipidemia (272).
We identified outcomes through ICD-9-CM codes 410–411 for acute MI, stroke, codes 430–438, and revenue (emergency) codes 450–459 for MI.11,12 We excluded such outcomes that occurred less than two weeks following the first TZD prescription; because of the proximity of these outcomes to the first TZD drug exposure, they would not have been associated with drug effects. Typically, such a cutoff point occurs 30 days after the initiation of a new drug therapy; in our study, however, we used 14-day cutoff points after we examined the data set. We based the 14-day cutoff point on our observation that after seven days, the pattern in the frequency of CV incidents among the same patient populations began to change and showed no CV incidents for another seven days. A number of events began to re-emerge gradually after 14 days of the initiation of the TZDs. In comparison, the pause in the rate of CV events was not present in the OAD group in the data set. Our main interest was the first incidence of a CV event after the initiation of TZD treatment.
We used SAS version 9.1 (SAS Institute, Cary, N.C.) and Stata version 9.2 software (Stata Corp., College Station, Tex.) to manage the data and to conduct our statistical analyses.
RESULTS
Sample Characteristics
Table 1 shows the baseline characteristics of the study sample by the type of drug (OAD or TZD). Both groups of patients were assumed to be at high risk because they had been taking at least one OAD from the starting point of our observation. The sample’s baseline characteristics suggested that:
African-Americans and patients using tobacco, alcohol, or drugs were less likely to receive prescriptions for TZDs compared with all other groups.
Patients ranging from 40 to 65 years of age who also had hypertension or hyperlipidemia in addition to diabetes were more likely to be prescribed TZDs than were other patients with diabetes.
Patients with pre-existing heart conditions or those who were older than 65 years of age were less likely to be prescribed TZDs.
Table 1.
Baseline Characteristics of Patients with Type-2 Diabetes Mellitus Receiving Oral Antidiabetic Drugs (OADs)
| Characteristics (N = 14,623) | OAD Users (N = 8,911) | TZD Users (N = 5,712) | P Value |
|---|---|---|---|
| Sex* | 0.1043 | ||
| Male† | 2,894 (20) | 1,929 (13) | |
| Female | 6,017 (41) | 3,783 (26) | |
| Age at Jan. 1, 2007 | <0.0001 | ||
| • Age below 40 years | 1,581 (11) | 1,114 (8) | |
| •Age between 40 and 65 years | 6,392 (44) | 4,113 (28) | |
| •Age over 65 years | 938 (6) | 485 (3) | |
| Race | <0.0001 | ||
| •Caucasian | 2,830 (19) | 2,111 (14) | |
| •African-American | 5,417 (37) | 3,087 (21) | |
| •Other | 664 (5) | 514 (4) | |
| Comorbidity | |||
| •Hypertension | 7,176 (49) | 4,484 (31) | 0.0029 |
| •Hyperlipidemia | 5,729 (39) | 3,598 (25) | 0.1102 |
| •Pre-existing heart condition | 229 (2) | 140 (1) | 0.6547 |
| •Obesity | 2,759 (19) | 1,672 (11) | 0.03 |
| •Drug, substance, alcohol, tobacco use | 2,036 (14) | 1,090 (7) | <0.0001 |
| Place of residency | <0.0001 | ||
| •Baltimore City | 3,821 (26) | 1,852 (13) | |
| •Other | 5,090 (35) | 3,860 (26) | |
Rows do not add up to 100% because patients may have multiple indications and risk factors. Percentages by sex, race, place of residency, and age group are mutually exclusive and add up to 100%.
Percentage figures are in parentheses.
TZDs = thiazolidinediones.
Adjustment for Confounding Factors
In our sample, risk factors were distributed among treatment groups after the patients were stratified by quintiles of the propensity score. The balancing of covariates in each stratum removed their confounding effects in the sample. In the propensity score model, predicting TZD treatment to adjust for comorbidity conditions at baseline using a logistic function, we defined “comorbidities” as binary variables. We considered only comorbidities for the adjustment before the first TZD claim. Before and after the initially prescribed TZD treatment, we found that new comorbidities rarely developed in our data set after the filing of the TZD claim.
Within each of the five strata, we calculated the difference-in-means of covariates for patients receiving at least one OAD. This process was designed to test whether the covariate means of patients in the OAD group differed from those in the TZD group within each stratum. Using a weighted average over the five strata, we conducted repeated tests of a t-statistic of difference-in-means between the treatment groups and each covariate. Student’s t-test of equality showed no statistically significant difference in any of the strata at the 5% significance level; thus, the distribution of covariates could be functionally considered random between the two treatment groups within each stratum.
No difference was observed at the 10% significance level; therefore, results displayed were at the 5% significance level. Thus, through the propensity score methods, we addressed the issue of channeling bias.
Logistic Regression Analysis
Table 2 shows results of the logistic regression predicting CV events based on TZD use. Table 3 represents the regression analysis using two TZDs as a separate explanatory variable. The base group in these analyses was represented by a Caucasian man with type-2 diabetes, younger than 40 years of age, and a nonresident of Baltimore with no other recorded comorbidities.
Table 2.
Logistic Regression Analysis of Cardiovascular Events (Dependent Variable) and Predictors (Independent Variables)
| Variable | Odds Ratio | Standard Error | P Value | 95% CI* | |
|---|---|---|---|---|---|
| TZD use | 1.009 | 0.054 | 0.864 | 0.909 | 1.121 |
| Female | 1.131 | 0.054 | 0.022 | 1.018 | 1.256 |
| African-American | 0.949 | 0.043 | 0.158 | 0.850 | 1.060 |
| Other race | 0.750 | 0.067 | 0.009 | 0.610 | 0.921 |
| Age (40–65 years) | 3.935 | 0.047 | <0.0001 | 3.141 | 4.929 |
| Age (65+ years) | 5.172 | 0.061 | <0.0001 | 3.988 | 6.706 |
| Hypertension | 1.608 | 0.277 | 0.087 | 0.934 | 2.769 |
| Hyperlipidemia | 0.405 | 0.476 | 0.057 | 0.159 | 1.028 |
| Pre-existing heart condition | 4.260 | 0.110 | <0.0001 | 3.431 | 5.289 |
| Obesity | 0.797 | 0.111 | 0.041 | 0.640 | 0.991 |
| Drug, tobacco or alcohol use | 3.170 | 0.239 | <0.0001 | 1.705 | 4.347 |
| Baltimore City residency | 3.041 | 0.387 | 0.004 | 1.425 | 6.490 |
TZD = thiazolidinedione.
95% confidence interval (CI) for odds ratio.
Table 3.
Logistic Regression Analysis: Separate Thiazolidinedione Drug Effects and Other Covariates Regressed on Adverse Cardiovascular Events Using the Propensity Score
| Variable | Odds Ratio | Standard Error | P Value | 95% CI* | |
|---|---|---|---|---|---|
| Rosiglitazone use | 1.124 | 0.054 | 0.032 | 1.010 | 1.250 |
| Pioglitazone use | 1.031 | 0.068 | 0.653 | 0.902 | 1.179 |
| Female | 1.127 | 0.054 | 0.025 | 1.015 | 1.252 |
| African-American | 0.953 | 0.043 | 0.142 | 0.853 | 1.064 |
| Other races | 0.751 | 0.067 | 0.009 | 0.611 | 0.922 |
| Age (40–65 years) | 3.915 | 0.047 | <0.0001 | 3.125 | 4.904 |
| Age (65+ years) | 5.163 | 0.061 | <0.0001 | 3.982 | 6.694 |
| Hypertension | 1.605 | 0.277 | 0.088 | 0.932 | 2.763 |
| Hyperlipidemia | 0.402 | 0.476 | 0.055 | 0.158 | 1.021 |
| Pre-existing heart condition | 4.266 | 0.111 | <0.0001 | 3.435 | 5.297 |
| Obesity | 0.792 | 0.112 | 0.036 | 0.636 | 0.985 |
| Drug, tobacco, or alcohol use | 2.725 | 0.239 | <0.0001 | 1.706 | 4.351 |
| Baltimore City residency | 3.033 | 0.387 | 0.004 | 1.421 | 6.473 |
95% confidence interval (CI) for odds ratio.
Pioglitazone and rosiglitazone use was also defined by cumulative exposure to either drug for more than 60 days. The mean exposure was 173 days for a patient receiving a TZD. Table 2 shows no evidence that cumulative exposure to an individual TZD (or the TZD drug class) over a period of 60 days increased the risk of adverse CV events at the 5% significance level when we controlled for confounding factors. When we included the exposure to each TZD drug in the regression as a separate explanatory variable after controlling for confounding factors, cumulative exposure to rosiglitazone for more than 60 days increased the risk of a CV event by 20% at the same level. Pioglitazone use did not show an increasing risk of such an event at conventional significance levels.
Comorbidities such as pre-existing heart conditions and drug, alcohol, or tobacco use increased the likelihood of an adverse CV event at the 5% significance level in both regression models. Obesity, hypertension, and hyperlipidemia were not significant factors in increasing the risk of the adverse CV events (P = 0.05). It is possible that some of these comorbidities were underreported, given their commonly known epidemiology. For example, Sachdev et al. reported that 60% of people 60 years of age and older have two or more chronic illnesses.13 In a different logistic regression model, testing TZD doses (of pioglitazone and rosiglitazone) as separate, dependent variables showed that the doses did not significantly increase the risk of adverse CV events at conventional significance levels.
Among the demographic characteristics, patient ages between 40 and 65 years and ages older than 65 years were associated with a significantly increased risk of an adverse event at the 5% level. Belonging to the “all others” group significantly reduced the risk of a CV event at conventional levels. It is difficult to explain the reduced risk for this group. Having a city residence (Baltimore) increased the risk by at least 60% and by 11% independently at the 10% significance level.
As shown in Table 4, we removed nonsignificant variables and tested “goodness of fit.” We used rosiglitazone; female sex; age (combining the two groups and re-running the regression); pre-existing heart disease; drug, alcohol, or tobacco use; Baltimore residence; race; and obesity. We performed the Hosmer–Lemeshow goodness-of-fit test, and the model fit the data well.
Table 4.
Logistic Regression Analysis: Rosiglitazone Effects and Significant Covariates Regressed on Adverse Cardiovascular Events
| Variable | Odds Ratio | Standard Error | P Value | 95% CI* | |
|---|---|---|---|---|---|
| Rosiglitazone use | 1.20 | 0.05 | 0.00070 | 1.08 | 1.33 |
| Female | 1.16 | 0.05 | 0.00540 | 1.05 | 1.29 |
| African-American | 0.90 | 0.04 | 0.01460 | 0.83 | 0.98 |
| Age (40+ years) | 5.76 | 0.11 | <0.0001 | 4.62 | 7.18 |
| Pre-existing heart condition | 4.79 | 0.11 | <0.0001 | 3.86 | 5.94 |
| Obesity | 1.13 | 0.05 | 0.02820 | 1.01 | 1.25 |
| Drug or alcohol use | 1.50 | 0.06 | <0.0001 | 1.34 | 1.67 |
| Baltimore City residency | 1.13 | 0.05 | 0.00500 | 1.01 | 1.25 |
95% confidence interval (CI) for odds ratio.
Goodness-of-fit: 0.6293.
Sensitivity Analysis
We performed sensitivity analyses using a different outcome variable and after excluding patients who had been treated with insulin. We first conducted the analysis, then ran the same regression. We found no evidence that TZD use increased the likelihood of an adverse event (P = 0.05). Results of this analysis are presented in Table 5.
Table 5.
Sensitivity Analysis Excluding Patients Using Insulin
| Variable | Odds Ratio | Standard Error | P Value | 95% CI* | |
|---|---|---|---|---|---|
| TZD use | 1.036 | 0.059 | 0.547 | 0.923 | 1.163 |
| Female | 1.091 | 0.058 | 0.136 | 0.973 | 1.223 |
| African-American | 0.956 | 0.046 | 0.299 | 0.847 | 1.079 |
| Other races | 0.791 | 0.071 | 0.046 | 0.635 | 0.984 |
| Age (40–65 years) | 3.730 | 0.052 | <0.0001 | 2.901 | 4.796 |
| Age (65+ years) | 4.929 | 0.066 | <0.0001 | 3.701 | 6.566 |
| Hypertension | 1.911 | 0.303 | 0.032 | 1.057 | 3.458 |
| Hyperlipidemia | 0.480 | 0.522 | 0.159 | 0.172 | 1.334 |
| Pre-existing heart condition | 4.110 | 0.124 | <0.0001 | 3.223 | 5.240 |
| Obesity | 0.783 | 0.122 | 0.046 | 0.616 | 0.996 |
| Drug, alcohol, or tobacco use | 2.605 | 0.262 | 0.000 | 1.560 | 4.348 |
| Baltimore City residency | 2.522 | 0.422 | 0.028 | 1.103 | 5.764 |
TZD = thiazolidinedione.
95% confidence interval (CI) for odds ratio.
In the analysis, those who were 40 years of age and older had a significantly increased risk of an adverse event. Pre-existing heart conditions and drug, alcohol, or tobacco use also increased the risk by more than two-fold. City residence and hypertension were also associated with an elevated risk of a CV event (P = 0.05).
By limiting the outcome of the adverse events to cases of acute MI, we also conducted logistic regression analyses. In these analyses, pre-existing heart conditions increased the risk of having an adverse event (P = 0.10). We did not find any evidence that exposure to TZDs over a period of 60 days increased the risk of a CV event at the 5% significance level. In the regression model, differentiating between exposures to the two individual TZDs, we also found no evidence of an increased risk of a CV event. The sample had small numbers of MI cases (five in the TZD arm and five in the OAD cohort).
DISCUSSION
Our study provided an examination of each antidiabetic drug available on the market as well as a drug class effect of TZDs on CV events of patients with type-2 diabetes mellitus in a high-risk, largely minority population, generally underrepresented in other TZD drug trial research or in the published literature. Randomized, controlled clinical trials cannot answer all of the important questions about drug effects in the general population. Typically, the main limitations are that participants in trials tend to be healthier and the data tend to rely on a relatively short duration of exposure to a drug and on a short observation period.14
More susceptible patients, such as the elderly or high-risk groups, are usually underrepresented in these trials. For these reasons, observational studies are more suitable for detecting rare or late adverse treatment effects and for providing opportunities to learn what outcomes are achieved in daily medical practice.15 It is possible that generalizations from trials can sometimes be misleading. Effect size, baseline risks, and co-morbidities may differ between trial participants and the broader population, which is not represented in the trials.16
In this context, our study compared the risk of an adverse CV event between TZD and other OAD regimens in patients with type-2 diabetes in a high-risk Medicaid population. Because the previous meta-analyses presented various results regarding TZD effects, these meta-analyses appear to have their own limitations. It is noteworthy that the decline in sales of TZDs has not resulted in a higher number of prescriptions for other OADs.17 This perhaps indicates a cautionary behavior of patients (and the treating physicians) in choosing OADs after the adverse effects of the drug were reported. Given that information in our data set was collected only until June 2006, well before the publication of more recent studies (January–May 2007) on the potential dangerous effect of the TZDs, our observational study is less likely to be biased, in that it was based on a change in behavior of patients and physicians.
Our results complement, and also contradict, some previous findings. Nissen and Wolski documented a CV risk associated with the use of rosiglitazone.1 Specifically, rosiglitazone increased the risk of MI. These authors also found a moderate significance associated with an increased risk of mortality from CV causes. They drew their conclusions from a meta-analysis of 42 clinical trials; a total of 116 trials screened from publicly available FDA data, a clinical trials registry from the drug manufacturer, and other published literature.
One major limitation of those studies was that the trials reviewed were not originally intended to explore CV events. Therefore, attention was not directed toward CV outcomes. Furthermore, definitions for MI were not provided. There was also considerable uncertainty surrounding the risk ratio because of the small sample sizes present to detect MI and death from CV causes.
Singh et al. also found that rosiglitazone increased the risk of MI and heart failure without significantly raising the risk of mortality caused by CV events.4 This study was also a meta-analysis that consisted of only four long-term, randomized, controlled trials that reviewed CV events associated with rosiglitazone.
STUDY LIMITATIONS
Our study included the same limitations concerning the sample size as those in the Nissen and Wolski study.1 Those authors recommended an analysis of a larger population with a longer-follow-up time to provide more definitive answers about CV mortality rates and the use of rosiglitazone. Our study confirmed that rosiglitazone resulted in an increased risk of MI and stroke by 20% at the 5% significance level. This rate was comparable to the findings in a population-based study of older patients by Lipscombe et al.18 However, we did not find sufficient evidence that TZDs as a drug class increased the risk of a CV event in this high-risk population. One possible explanation is that our study population tended to be younger and was not typical of patients seen in randomized clinical trials. As stated earlier, our population consisted mostly of females and an African-American study sample, in which approximately one-third were from the city of Baltimore.
In a separate regression, our study also demonstrated that rosiglitazone use was associated with an increase in CV events, whereas pioglitazone was not. Our study supported previous meta-analysis trials and further suggested that female sex, age, and pre-existing heart conditions might be strong predictors of CV events. Interestingly enough, obesity was a CV protective factor. Because our study already controlled for preconditions like hypertension, hyperlipidemia, or a pre-existing heart condition, this finding suggested that obesity itself (not its related comorbidities) might not be a predictor of CV events in this case.
TZD doses were not significantly associated with an increased risk of CV events. Although Nissen and Wolski1 and Lincoff et al.19 suggested that pioglitazone lowered the risk of death, MI, or stroke among a diverse population of patients with diabetes, our study showed no evidence that pioglitazone reduced the likelihood of a CV event (MI or stroke) in the Medicaid population. Therefore, it is difficult for us to suggest that the insufficient evidence of an increased risk of TZD drug class effects resulted from two opposite drug effects in the same class. When rosiglitazone increases the risk, as pioglitazone decreases the risk, the offsetting of these opposite drug effects would be anticipated. Instead, we did not establish evidence that pioglitazone lowered the risk of a CV event in a high-risk population.
Another study appeared to provide a possible explanation for our findings. Türkemen et al. found that in patients with type-2 diabetes, “TZD treatment might have slight adverse effects on ventricular contractility and fluid dynamics at the beginning of the therapy. However, these changes seem to stabilize in the long term.”20 To further test and confirm this explanation, it might be useful to analyze the comparative or compatible samples using Medicare and Medicaid data sets from other states with a similar demographic distribution of patient populations.
Several limitations were inherent in our study.
First, although our propensity score–matching method significantly reduced bias, there could still be residual bias.21 Further, we used five strata in propensity scoring, and the difference in mean of covariates was not statistically significant to maximize the possibility that the distribution of covariates could be practically considered random between the two treatment groups. However, important clinical differences might still exist, even though statistical significance was not reached. We might be missing some critical, unobserved variables or risk factors for CV outcomes (e.g., unreported smoking).
Second, we used inclusion and exclusion criteria to define exposure to drugs and to identify the appropriate sample for our research. This process can help us better characterize our study sample, but it is also possible that we proceeded with certain types of selection bias with sampling when applying the exclusion and inclusion criteria of our study. We believe that propensity score methods and the discussions of sensitivity tests included in the study address that issue.
Because of the nature of common claims data, we do not have accurate information to control for severity of disease. It is not clear whether our findings represent only drug effects or the severity of disease effects. Also, ICD-9 codes were used to identify diseases; therefore, our study is subject to possible misclassification bias.
We did not explain the change in prescribing patterns. If doctors were aware of the research findings regarding rosiglitazone’s adverse effect during our observation period, this could have changed the prescription patterns and the study results. Although it is unlikely that a major shift in the prescription pattern occurred before July 2006 (the date of the first publication was May 2007), it is possible that physicians became aware of the risks of TZD prescribing for diabetic patients. This awareness could have contributed to the change in prescribing patterns.
Our study did not include a placebo group as a reference group. We did not distinguish specific TZD drug effects from those in a placebo-treated control group. Interpretations of studies involving active and multiple drug comparator groups differ from those related to placebo-controlled analyses.
Finally, we might have introduced confounding by comedication bias into the data set. We did not control for this factor because our study used an active multiple-drug comparator group as a reference group. TZDs are frequently used in combination with other antidiabetic drugs.
CONCLUSION
Our study contributes to the literature by showing a difference in the rate of CV events associated with rosiglitazone and pioglitazone in a high-risk, largely African-American and under-represented Medicaid population. Our findings suggest that physicians should use great caution when prescribing rosiglitazone to high-risk patients with type-2 diabetes mellitus.
Figure 3.
Cohort follow-up. OAD = oral diabetic drug; TZD = thiazolidinedione.
Acknowledgments
Author Contributions and Acknowledgments: K. Sohn had full access to all of the data during the study, under the supervision of F. Shaya, and assumes responsibility for the integrity of the data and the accuracy of the data analysis. F. Shaya supervised the study and oversaw the study concept and design and the acquisition of data. F. Shaya and K. Sohn analyzed and interpreted the data. F. Shaya, K. Sohn, and Confidence M. Gbarayor drafted the manuscript. F. Shaya and M. Weir performed a critical revision of the manuscript for intellectual content. F. Shaya, K. Sohn, and Z. Lu performed the statistical analysis.
Footnotes
Disclosure: The authors have no financial or commercial relationships to report with regard to this article.
REFERENCES
- 1.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM. Rosiglitazone and cardiotoxicity: Weighing the evidence. N Engl J Med. 2007;357(1):64–66. doi: 10.1056/NEJMe078117. [DOI] [PubMed] [Google Scholar]
- 3.Volkova N, McClellan W, Soucie M, Schoolwerth A. Racial disparities in the prevalence of cardiovascular disease among incident end-stage renal disease patients. Nephrol Dial Transplant. 2006;21(8):2202–2209. doi: 10.1093/ndt/gfl078. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: A meta-analysis. JAMA. 2007;298(10):1189–1195. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Loke YK, Furberg CD. Thiazolidinediones and heart failure: A tele-analysis. Diabetes Care. 2007;30(8):2148–2153. doi: 10.2337/dc07-0141. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 7.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 8.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8S):757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 9.Hartung DM, Touchette DR, Bultemeier NC, Haxby DG. Risk of hospitalization for heart failure associated with thiazolidinedione therapy: A Medicaid claims-based case–control study. Pharmacotherapy. 2005;25(10):1329–1336. doi: 10.1592/phco.2005.25.10.1329. [DOI] [PubMed] [Google Scholar]
- 10.Delea TE, Edelsberg JS, Hagiwara M, et al. Use of thiazolidinediones and risk of heart failure in people with type 2 diabetes: A retrospective cohort study. Diabetes Care. 2003;26(11):2983–2989. doi: 10.2337/diacare.26.11.2983. [DOI] [PubMed] [Google Scholar]
- 11.Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144(2):290–296. doi: 10.1067/mhj.2002.123839. [DOI] [PubMed] [Google Scholar]
- 12.Madsen M, Davidsen M, Rasmussen S, et al. The validity of the diagnosis of acute myocardial infarction in routine statistics: A comparison of mortality and hospital discharge data with the Danish MONICA registry. J Clin Epidemiol. 2003;56(2):124–130. doi: 10.1016/s0895-4356(02)00591-7. [DOI] [PubMed] [Google Scholar]
- 13.Sachdev M, Sun JL, Tsiatis AA, et al. The prognostic importance of comorbidity for mortality in patients with stable coronary artery disease. J Am Coll Cardiol. 2004;43(4):576–582. doi: 10.1016/j.jacc.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Stürmer T, Glynn RJ, Rothman KJ, et al. Adjustments for unmeasured confounders in pharmacoepidemiologic database studies using external information. Med Care. 2007;45(10 Suppl 2):S158–S165. doi: 10.1097/MLR.0b013e318070c045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elm EV, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann Intern Med. 2007;147(8):573–578. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 16.Strom BL, editor. Pharmacoepidemiology. 4th ed. Sussex, England: John Wiley & Sons Ltd; 2005. [Google Scholar]
- 17.Saul S. VA limits Glaxo drug widely used for diabetes. The New York Times. Oct 18, 2007.
- 18.Lipscombe LL, Gomes T, Lévesque LE, et al. Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. JAMA. 2007;298(22):2634–2643. doi: 10.1001/jama.298.22.2634. [DOI] [PubMed] [Google Scholar]
- 19.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. JAMA. 2007;298(10):1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 20.Türkmen KY, Güvener DN, Yildirir A, et al. Effects of rosiglitazone on plasma brain natriuretic peptide levels and myocardial performance index in patients with type 2 diabetes mellitus. Acta Diabetol. 2007;44(3):149–156. doi: 10.1007/s00592-007-0256-4. [DOI] [PubMed] [Google Scholar]
- 21.Bloom HS, Hill CJ. Can Propensity-score methods match the findings from a random assignment evaluation of mandatory welfare-to-work programs? Rev Economics Statistics. 2004;186(186):156–179. [Google Scholar]