Abstract
The contributions of donor kidney quality (partially determined by donor age), allograft rejection, and calcineurin inhibitor nephrotoxicity on the progression of histologic damage of renal allografts are not completely defined. Moreover, the determinants of individual susceptibility to calcineurin inhibitor nephrotoxicity are not known but may include variability in drug transport and metabolism. In a prospective cohort of 252 adult renal allograft recipients treated with a combination of tacrolimus, mycophenolate mofetil, and corticosteroids, we studied 744 renal allograft biopsies obtained regularly from time of transplantation for 3 yr. We assessed determinants of histologic evolution, including tacrolimus exposure, renal P-glycoprotein (ABCB1) expression, and polymorphisms in the CYP3A4, CYP3A5, and ABCB1 genes. Within the first 3 yr after transplantation, we noted a progressive increase in interstitial fibrosis, tubular atrophy, glomerulosclerosis, and vascular intimal thickening. Older donor age, absence of P-glycoprotein expression at the apical membrane of tubular epithelial cells, and combined donor–recipient homozygosity for the C3435T variant in ABCB1 significantly associated with increased susceptibility to chronic allograft damage independent of graft quality at implantation. Changes in graft function over time reflected these associations with donor age and ABCB1 polymorphisms, but it was acute T cell-mediated and antibody-mediated rejection that determined early graft survival. In conclusion, the effects of older donor age reach beyond the quality of the allograft at implantation and continue to be important for histologic evolution in the posttransplantation period. In addition, ABCB1 genotype and expression of P-glycoprotein in renal tubular epithelial cells determine susceptibility to chronic tubulointerstitial damage of transplanted kidneys.
Progressive renal allograft dysfunction resulting from cumulative histologic damage to the allograft is the major cause of late renal allograft loss after recipient death with a functioning graft.1,2 The evolution of renal allograft histology therefore can be regarded as a valuable surrogate marker for long-term graft outcome.3 This evolution has been described in detail by Nankivell et al. using renal allograft biopsies obtained at preset time points after transplantation in kidneys of pristine quality at implantation.4 In this study, the kidneys were recovered from a selected group of relatively young donors, and the majority of recipients (kidney–pancreas transplants in all but 1) were treated with a combination of the older formulation of cyclosporine in combination with azathioprine and corticosteroids.4
However, with the increasing use of kidneys from older or extended criteria donors for transplantation, poor graft quality at implantation emerges as an important determinant of long-term outcome.5,6 Therefore, the experience of Nankivell et al. may no longer be representative for current clinical practice. In addition, immunosuppressive drug combinations have improved over the past few decades,7,8 and this has an impact on both histologic and functional evolution of allografts.9–11 On one hand, although the newer immunosuppressive protocols have reduced the incidence of acute cellular rejection, rejection phenomena continue to play a major role in this histologic evolution. On the other hand, immunosuppressive drugs can elicit direct (e.g., nephrotoxicity of calcineurin inhibitors) and indirect (diabetes mellitus, hyperlipidemia, and hypertension) side effects, which also contribute to renal allograft injury.12,13
The complementary impact of these phenomena (donor kidney quality, allograft rejection, and calcineurin inhibitor nephrotoxicity) on the progression of histologic allograft damage has not been studied in patients treated with the current powerful immunosuppressive protocols nor within the wide range of donor graft quality. Moreover, the determinants of individual susceptibility to calcineurin inhibitor nephrotoxicity are not well known. Previous animal and human studies have suggested a role of decreased P-glycoprotein (ABCB1 or MDR1, the multidrug efflux transporter involved in tacrolimus transport14) expression15–17 or activity18 and genetic polymorphisms in ABCB119,20 in the development of calcineurin inhibitor nephrotoxicity.
The current study was undertaken to assess the determinants of the histologic evolution of renal allografts in the first years after transplantation in 252 patients treated with a combination of tacrolimus, mycophenolate mofetil, and corticosteroids against a background of varying degrees of pre-existing histologic damage in the donor kidney at implantation.8,11 In addition, the determinants of individual susceptibility to calcineurin inhibitor nephrotoxicity were evaluated, including extensive tacrolimus exposure data, P-glycoprotein expression, and polymorphisms in ABCB1, CYP3A4, and CYP3A5 of both donor and recipients. Finally, this study examined the features that predict lower MDRD glomerular filtration rate during follow-up and assessed the main determinants of early graft survival.
Results
Study Population Characteristics.
Patient and donor demographics and transplantation-related characteristics are summarized in Table S1. The study group consisted of 252 consecutive adult renal allograft recipients who received a single kidney at the University Hospitals Leuven between 2004 and 2007 and were treated with an immunosuppressive regimen consisting of tacrolimus (Prograft, Astellas) in combination with mycophenolate mofetil (CellCept, Roche) and oral methylprednisolone (Medrol, Pfizer). Recipients were 54.5 ± 13.9 yr of age, and 62.3% were male. Mean donor age was 46.7 ± 15.1 yr, and 58.3% were male. Ninety-three percent of kidneys were obtained from deceased donors; stroke was the reason of death in 52.8%. Ninety-seven patients with higher immunologic risk (second or third transplantation, prior sensitization, young recipient age, black recipient race, and living donor kidneys) received induction therapy with IL-2 receptor blocking monoclonal antibodies (n = 85) or anti-T cell immunoglobulins (n = 12). All patients with clinical and subclinical Banff type I or II–III acute cellular rejection21,22 were treated with high doses of methylprednisolone in a tapering protocol. No treatment adjustments were made for the appearance or progression of chronic histologic lesions. Written informed consent was obtained from all patients, and the study was approved by the institutional review board and ethics committee.
The daily tacrolimus dose was adjusted to achieve target predose blood concentrations between 12 and 15 ng/ml in the first 3 mo after transplantation. From 3 to 12 mo, doses were adjusted to achieve predose concentrations of 9 to 12 ng/ml. Thereafter, a target range of 8 to 10 ng/ml was maintained. All tacrolimus predose trough (C0) levels determined during follow-up were included in the analysis (n = 14,125). In addition, at 3, 12, 24, and 36 mo after transplantation, tacrolimus pharmacokinetic profiles were obtained using abbreviated 4-h time concentration profiles. The evolution (“maturation”) of tacrolimus pharmacokinetics is summarized in Table S2.
DNA (extracted from whole blood samples) was available for analysis from 250 recipients and 239 donors. Single-nucleotide polymorphisms of ABCB1 (C3435T and G2677T/A), CYP3A4 (A-290G; CYP3A4*1 versus CYP3A4*1b), and CYP3A5 (G6986A; CYP3A5*1 versus CYP3A5*3) were determined for all 489 DNA samples. All single-nucleotide polymorphisms were in Hardy–Weinberg equilibrium (Table S3). There was a significant linkage disequilibrium between CYP3A4*1b and CYP3A5*1 and between ABCB1 C3435T and G2677T/A (Figure S1 and Table S4). Polymorphisms in CYP3A4 and CYP3A5 of recipients were associated significantly with tacrolimus pharmacokinetics; polymorphisms in ABCB1 did not have any impact on tacrolimus pharmacokinetics (Table S2).
Kidney biopsies were performed routinely (“protocol biopsies”) at the time of transplantation (before reperfusion) and at 3, 12, 24, and 36 mo. In addition, “indication biopsies” were performed at times of renal allograft dysfunction. A total of 744 biopsies were available, after exclusion of five biopsies with insufficient tissue: 179 indication biopsies (159 within the first 3 mo and 20 after 3 mo) and 565 protocol biopsies (Tables S5 and S6). Nine protocol biopsies were excluded from the analyses because the immunosuppressive regimen was changed before the biopsy, leaving 556 protocol biopsies in the analyses.
Implantation Biopsies.
Fifty-four percent (98 of 179) of patients received a kidney of pristine condition (absence of interstitial fibrosis/tubular atrophy [IF/TA grade 0], absence of vascular intimal thickening, absence of arteriolar hyalinosis, and absence of glomerulosclerosis), assessed in biopsies performed at implantation or within the first 2 wk after transplantation in case no implantation biopsy was available. Donor age was the only risk factor independently associated with IF/TA (odds ratio [OR] 1.10 [1.05 to 1.14] per year, P < 0.001), glomerulosclerosis (OR 1.08 [1.04 to 1.11] per year, P < 0.001), and arteriolar hyalinosis (OR 1.04 [1.0 to 1.07] per year, P < 0.05) in implantation biopsies. Cold ischemia time was not associated with the histologic appearance of the kidneys at the time of transplantation nor with acute tubular necrosis (Table S7). Seventy-six implantation biopsies had frozen tissue stored for C4d staining; none of them showed C4d positivity at the peritubular capillaries.
Acute Histologic Changes after Transplantation.
Indication biopsies were performed mainly within the first 3 mo after transplantation (159 of 179; 88.8%) and showed acute cellular rejection in 37.1% (59 of 159) of cases, representing 20.2% (51 of 252) of patients. Forty percent of late (after 3 mo) indication biopsies (8 of 20) had acute cellular rejection. The prevalence of subclinical acute rejection in protocol biopsies was highest at 3 mo (17 of 207; 8.21%) and decreased significantly by time after transplantation (Table 1). Acute antibody-mediated rejection (grade 1 to 3) was seen in 44 of 179 (24.6%) of the indication biopsies (24 of 252 patients [9.5%] had antibody-mediated rejection within the first 3 mo after transplantation) and in 18 of 423 (4.26%) of posttransplant protocol biopsies (Table 1). In protocol biopsies, there was no change in the prevalence of acute antibody-mediated rejection over time after transplantation (Table 1). Due to the low number of patients who developed light microscopic transplant glomerulopathy in the current study (14 of 735 posttransplant biopsies; 1.90%) and because none of these biopsies had C4d positivity in peritubular capillaries, chronic antibody-mediated rejection was not detected (Table 1). There was a significant association between acute cellular rejection and acute antibody-mediated rejection, both in indication biopsies and in 3-mo protocol biopsies: 24 of 67 (35.8%) of indication biopsies with acute cellular rejection also had antibody-mediated rejection, versus 20 of 112 (17.9%) of acute-cellular-rejection-free biopsies, P = 0.007; 3 of 17 (17.7%) 3-mo protocol biopsies with acute rejection had antibody-mediated rejection versus 7 of 190 (3.68%) 3-mo acute-cellular-rejection-free protocol biopsies, P = 0.039.
Table 1.
Histological evolution of protocol biopsies obtained at implantation and at 3, 12, 24, and 36 mo after transplantation
| Implantation | 3 mo | 12 mo | 24 mo | 36 mo | P value | ||
|---|---|---|---|---|---|---|---|
| Theoretical number of eligible protocol biopsies | 252 | 252 | 184 | 109 | 53 | ||
| Actual number of protocol biopsies included in the study | 133 | 207 | 140 | 55 | 21 | ||
| Acute T cell-mediated rejection | Absent | 133 (100%) | 160 (77.3%) | 124 (88.6%) | 53 (96.4%) | 20 (95.2%) | <0.0001a |
| Borderline | 0 (0%) | 30 (14.49%) | 12 (8.57%) | 1 (1.82%) | 1 (4.76%) | ||
| Acute | 0 (0%) | 17 (8.21%) | 4 (2.86%) | 1 (1.82%) | 0 (0%) | ||
| Acute antibody-mediated rejection | Absent | 75/76 (98.7%) | 189 (91.3%) | 130 (92.9%) | 49 (89.1%) | 19 (90.5%) | 0.8903a |
| Minimal C4d+ (<25%) | 1/76 (1.32%) | 8 (3.86%) | 6 (4.29%) | 2 (3.64%) | 2 (9.52%) | ||
| C4d+ (>25%) without evidence of active rejection | 0/76 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Acute antibody-mediated rejection grade 1 | 0/76 (0.0%) | 1 (0.48%) | 1(0.71%) | 0 (0.0%) | 0 (0.0%) | ||
| Acute antibody-mediated rejection grade 2 | 0/76 (0.0%) | 8 (3.86%) | 3 (2.14%) | 4 (7.27%) | 0 (0.0%) | ||
| Acute antibody-mediated rejection grade 3 | 0/76 (0.0%) | 1 (0.48%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Chronic antibody-mediated rejection | Absent | 76/76 (100%) | 207 (100%) | 140 (100%) | 55 (100%) | 21 (100%) | 1.0 |
| Present | 0/76 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Interstitial fibrosis (Banff “ci” score) | 0 to 5% | 96 (72.2%) | 148 (71.5%) | 67 (47.9%) | 16 (29.1%) | 5 (23.8%) | <0.0001 |
| 6 to 25% | 24 (18.1%) | 35 (16.9%) | 44 (31.4%) | 26 (47.3%) | 11 (52.4%) | ||
| 26 to 50% | 12 (9.02%) | 19 (9.18%) | 24 (17.1%) | 10 (18.2%) | 3 (14.3%) | ||
| >50% | 1 (0.75%) | 5 (2.42%) | 5 (3.57%) | 3 (5.45%) | 2 (9.52%) | ||
| Tubular atrophy (Banff “ct” score) | 0% | 71 (53.4%) | 71 (34.3%) | 9 (6.43%) | 1 (1.82%) | 1 (4.76%) | <0.0001 |
| <25% | 56 (42.1%) | 127 (61.4%) | 109 (77.9%) | 43 (78.2%) | 15 (71.4%) | ||
| 26 to 50% | 5 (3.76%) | 7 (3.38%) | 17 (12.1%) | 9 (16.4%) | 4 (19.1%) | ||
| >50% | 1 (0.75%) | 2 (0.97%) | 5 (3.57%) | 2 (3.64%) | 1 (4.76%) | ||
| Banff “IF/TA” grade | Grade 0 | 104 (78.2%) | 155 (74.9%) | 68 (48.6%) | 16 (29.1%) | 5 (23.8%) | <0.0001 |
| Grade 1 | 24 (18.1%) | 43 (20.8%) | 51 (36.4%) | 28 (50.9%) | 11 (52.4%) | ||
| Grade 2 | 5 (3.76%) | 7 (3.38%) | 16 (11.4%) | 9 (16.4%) | 4 (19.1%) | ||
| Grade 3 | 0 (0%) | 2 (0.97%) | 5 (3.57%) | 2 (3.64%) | 1 (4.76%) | ||
| Percentage globally sclerosed glomeruli | <25% | 126 (94.7%) | 201 (97.1%) | 138 (98.6%) | 53 (96.4%) | 18 (85.7%) | 0.0379 |
| >25% | 7 (5.26%) | 6 (2.90%) | 2 (1.43%) | 2 (3.64%) | 3 (14.3%) | ||
| Vascular intimal thickening (Banff “cv” score) | Absent | 131 (99.2%) | 137 (66.2%) | 78 (56.1%) | 34 (61.8%) | 15 (71.4%) | <0.0001 |
| Present with <25% luminal narrowing | 1 (0.76%) | 44 (21.3%) | 33 (23.7%) | 13 (23.6%) | 4 (19.1%) | ||
| Present with 25 to 50% luminal narrowing | 0 (0%) | 23 (11.1%) | 22 (15.8%) | 7 (12.7%) | 2 (9.52%) | ||
| Present with >50% luminal narrowing | 0 (0%) | 3 (1.45%) | 6 (4.32%) | 1 (1.82%) | 0 (0%) | ||
| Glomerular capsular fibrosis | Absent | 126 (95.5%) | 195 (94.2%) | 104 (74.3%) | 37 (67.3%) | 19 (90.5%) | <0.0001 |
| Present | 6 (4.55%) | 12 (5.8%) | 36 (25.7%) | 18 (32.7%) | 2 (9.52%) | ||
| Mesangial matrix increase (Banff “mm” score) | Absent | 127 (95.5%) | 187 (90.3%) | 127 (90.7%) | 45 (81.8%) | 20 (95.2%) | 0.0201 |
| Present | 6 (4.51%) | 20 (9.67%) | 13 (9.29%) | 10 (18.2%) | 1 (4.76%) | ||
| Transplant glomerulopathy (Banff “cg” score) | Absent | 131 (98.5%) | 207 (100%) | 134 (95.7%) | 52 (94.6%) | 18 (85.7%) | 0.0002 |
| Present | 2 (1.5%) | 0 (0%) | 6 (4.29%) | 3 (5.45%) | 3 (14.3%) | ||
| Tubular microcalcifications | Absent | 126 (94.7%) | 147 (71.0%) | 109 (77.9%) | 36 (65.5%) | 16 (76.2%) | 0.0003 |
| Present | 7 (5.26%) | 60 (29.0%) | 31 (22.1%) | 19 (34.6%) | 5 (23.8%) | ||
| Acute tubular necrosis | <5% of tubuli | 81 (60.9%) | 172 (83.1%) | 120 (85.7%) | 53 (96.4%) | 20 (95.2%) | <0.0001 |
| >5% of tubuli | 52 (39.1%) | 35 (16.9%) | 20 (14.3%) | 2 (3.64%) | 1 (4.76%) | ||
| Peripheral arteriolar hyalinosis (Banff “ah” score) | Absent | 111 (83.5%) | 148 (71.5%) | 98 (70%) | 40 (72.7%) | 17 (81.0%) | 0.1414 |
| Present | 22 (16.5%) | 59 (28.5%) | 42 (30%) | 15 (27.3%) | 4 (19.1%) | ||
| Peritubular capillaritis | Grade 0 | 132 (99.3%) | 184 (88.9%) | 124 (88.6%) | 47 (85.5%) | 17 (81.0%) | 0.9141a |
| Grade 1 | 1 (0.75%) | 10 (4.83%) | 14 (10%) | 5 (9.09%) | 3 (14.3%) | ||
| Grade 2 | 0 (0.0%) | 10 (4.83%) | 2 (1.43%) | 3 (5.45%) | 1 (4.76%) | ||
| Grade 3 | 0 (0.0%) | 3 (1.45%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| C4d deposition in peritubular capillaries | 0% of biopsy area | 75/76 (98.7%) | 189 (91.3%) | 130 (92.9%) | 49 (89.1%) | 19 (90.5%) | 0.1980a |
| 1 < 25% of biopsy area | 1/76 (1.32%) | 8 (3.86%) | 6 (4.29%) | 2 (3.64%) | 2 (9.52%) | ||
| 25 to 75% of biopsy area | 0/76 (0.0%) | 0 (0.0%) | 1 (0.71%) | 2 (3.64%) | 0 (0.0%) | ||
| >75% of biopsy area | 0/76 (0.0%) | 10 (4.83%) | 4 (2.14%) | 2 (3.64%) | 0 (0.0%) |
Already in the first 3 yr after transplantation, there was a rapid increase in interstitial fibrosis and tubular atrophy, which was present in more than 75% of biopsies performed at 3 yr after transplantation. In addition, there was a significant increase in the prevalence and extent of vascular intimal thickening by time after transplantation, mainly in the first 3 mo after transplantation. The prevalence of tubular microcalcifications increased only in the first 3 mo after transplantation. P values were obtained using Cochran–Mantel–Haenszel statistics.
aThe subclinical rejection evolution P value was calculated with exclusion of implantation biopsies.
Both in indication biopsies (graft dysfunction) and in protocol biopsies (stable graft function), acute T cell-mediated cellular rejection was associated with a higher number of HLA-DR mismatches (OR 4.48 [2.35 to 8.55], P < 0.001, and OR 4.08 [1.61 to 10.3], P = 0.003, for indication and protocol biopsies, respectively) (Tables S8 and S9). Acute antibody-mediated rejection in indication biopsies was associated with prior transplantation (OR 5.52 [2.19 to 14.1], P < 0.001) and with the use of a live donor kidney (OR 6.41 [1.88 to 21.7], P = 0.003) (Table S10). In protocol biopsies, the same association between antibody-mediated rejection and prior transplantation was observed (OR 6.67 [1.76 to 25.6], P = 0.005) (Table S11).
In addition, eight patients developed polyomavirus-associated nephropathy (PVAN) after transplantation; six patients with PVAN were treated with cidofovir. One of these grafts with PVAN was lost during follow-up. Seven patients developed focal sclerosis lesions (two of them recurrent original disease). Four other patients developed glomerular disease of the transplanted kidney: three recurrent disease (one patient light chain deposit disease and two patients IgA nephropathy) and one de novo membranous type glomerulonephritis.
Posttransplantation Evolution of Chronic Histologic Damage.
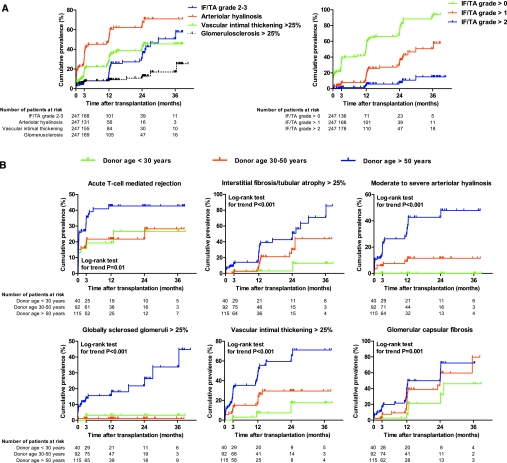
In the first 3 yr after transplantation, a gradual increase in chronic histologic damage of renal allografts was observed (Figure 1A and Table 1). Higher donor age was an independent risk factor for higher grades of IF/TA in posttransplant protocol biopsies (n = 423) (OR 3.3 [1.1 to 7.6], P = 0.03, and OR 11 [4.0 to 31], P < 0.001, for 30 to 50 versus <30 yr and >50 versus <30 yr, respectively) (Figure 1B and Table S12). Likewise, higher donor age was associated with new onset or progressive arteriolar hyalinosis. None of the kidneys from donors <30 yr developed moderate to severe arteriolar hyalinosis (Figure 1B and Table S13). A similar association was found between higher donor age and vascular intimal thickening, glomerulosclerosis, and glomerular capsular fibrosis in posttransplant protocol biopsies (Figure 1B and Tables S14 through S16). Stroke as the reason of donor death was associated with arteriolar hyalinosis, IF/TA, vascular intimal thickening, and global glomerulosclerosis in univariate analysis but was no longer retained in the final multivariate models after backward elimination, due to its dependency on donor age.
Figure 1.
Cumulative prevalence of chronic histologic lesions by time after transplantation. (A) Cumulative prevalence of different grades of IF/TA, arteriolar hyalinosis, vascular intimal thickening, and global glomerulosclerosis by time after transplantation, expressed as 1 − the Kaplan–Meier survival estimate. (B) Cumulative prevalence of T cell-mediated rejection, moderate to severe IF/TA, arteriolar hyalinosis, global glomerulosclerosis, vascular intimal thickening, and glomerular capsular fibrosis according to donor age groups, expressed as 1 − the Kaplan–Meier survival estimate. For the Kaplan–Meier estimates of event rates presented here, patients were censored at the time of their last biopsy. Five patients did not have any biopsy performed and were excluded from this analysis, leaving 247 patients in the analysis. The histologic lesions were scored according to the updated Banff classification21,22 on sequential biopsy specimens obtained on indication (graft dysfunction) and at prescheduled time points (protocol biopsies). IF/TA grade 1 is IF/TA encompassing <25% of the biopsy core, whereas grade 2 corresponds to 25 to 50% and grade 3 to IF/TA in >50% of the biopsy.
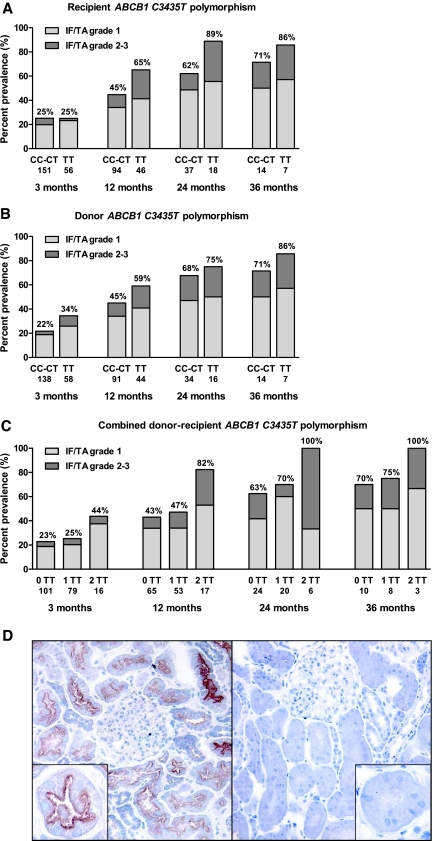
The association between the different histologic lesions and single-nucleotide polymorphisms in ABCB1, CYP3A4, and CYP3A5 was assessed in uni- and multivariate analyses (Tables S8 through S16). Both donor and recipient ABCB1 homozygosity for the variant allele T at position 3435 in exon 26 were associated with higher IF/TA grade in univariate analysis but not with other histologic lesions. In multivariate analysis, this association of the ABCB1 genotype with higher IF/TA grade was most prominent when donor and recipient were both homozygous for this common ABCB1 variant: The adjusted OR was 3.9 [2.0 to 7.6] (P < 0.001) if both donor and recipient were homozygous for the T variant versus no homozygosity for the C3435T polymorphism and OR 3.7 [1.8 to 7.7] (P < 0.001) for combined homozygosity of the T variant versus mixed homozygous–heterozygous combinations (Figure 2 and Table S12).
Figure 2.
P-Glycoprotein and the evolution of interstitial fibrosis and tubular atrophy. Impact of recipient (A) and donor (B) ABCB1 C3435T polymorphisms on IF/TA in the first 3 yr after transplantation. (C) Impact of combined donor and recipient ABCB1 C3435T genotype on IF/TA in the first 3 yr after transplantation. 0TT = no homozygosity for the TT variant (donor–recipient CC-CC, CC-CT, CT-CC, or CT-CT); 1TT = either donor or recipient homozygous for the TT variant (CC-TT, TT-CC, CT-TT or TT-CT); 2TT = both donor and recipient homozygous for the TT variant (TT-TT). (D) Immunohistochemical staining for P-glycoprotein on frozen sections of renal allograft biopsies at 3 mo after transplantation. Left: Positive staining for P-glycoprotein apically and at the brush border of tubular epithelial cells. Right: Negative staining for P-glycoprotein. Immunohistochemistry for P-glycoprotein was performed on cryostat sections (4-μm-thick), which were incubated with the primary monoclonal antibody (JSB-1 MmAb; dilution 1:10; Monosan) for 30 min at room temperature. The second and third step consisted of peroxidase-labeled rabbit anti-mouse and peroxidase-labeled swine anti-rabbit immunoglobulins (both from Dako Belgium N.V., Heverlee, Belgium). There was no association between P-glycoprotein gene expression and ABCB1 genotype (Table S18).
In univariate analysis, both clinical acute rejection in the first 3 mo (20.2% of patients) and subclinical acute rejection at 3 mo (8.2% of patients) after transplantation were associated with higher IF/TA grades in subsequent protocol biopsies (OR 2.1 [1.2 to 3.8], P = 0.01, and OR 4.3 [1.7 to 11], P = 0.002, respectively). These associations of clinical and subclinical rejection with IF/TA grade were no longer significant after multivariate backward elimination (Figure S2 and Table S12). Acute antibody-mediated rejection was not associated with the histologic lesions in subsequent protocol biopsies (IF/TA, glomerulosclerosis, arteriolar hyalinosis, vascular intimal thickening, and glomerular capsular fibrosis) (Tables S12 through S16). Due to the low prevalence of transplant glomerulopathy in the current study (which did not include electron microscopy data), it was not possible to analyze the previously described association between acute antibody-mediated rejection and this feature of chronic antibody-mediated rejection. Finally, parameters of tacrolimus exposure were neither associated with the chronic histologic appearance of renal allografts nor with an increased risk for clinical or subclinical acute cellular rejection (Tables S8 through S16).
After inclusion of donor graft quality in the multivariate model for IF/TA, donor age remained significant in the final model, independent of the significant impact of donor graft quality, the combined donor–recipient ABCB1 C3435T status, delayed graft function, and time after transplantation (Table S17). Likewise, when the analyses were repeated in the subgroup of patients who received kidneys in pristine condition (n = 98 patients), similar independent effects of donor age and combined donor–recipient ABCB1 polymorphisms were found on the evolution of IF/TA. Also in this group of recipients of pristine kidneys, a significant influence of donor age was found on posttransplant arteriolar hyalinosis, vascular intimal thickening, and glomerulosclerosis (data not shown).
Although CYP3A4 and CYP3A5 polymorphisms significantly impacted on tacrolimus pharmacokinetics (Table S2), this did not have an effect on rejection incidence or timing, or on histologic or functional evolution. ABCB1 polymorphisms also were not associated with a higher risk for acute rejection.
Immunohistochemistry for P-Glycoprotein.
A positive staining for P-glycoprotein was found in the majority of biopsies at 3 mo after transplantation (n = 64 of 76 biopsies), localized at the apical surface and brush border of both proximal and distal tubular epithelial cells (Figure 2D). One biopsy had only weak positive staining of the distal tubular epithelial cells without positivity of the proximal tubular epithelial cells and was regarded as negative. There was neither cytoplasmic staining nor staining in arterial endothelia. Some 3-mo biopsies had a negative stain for P-glycoprotein at the apical membrane of tubular epithelial cells (n = 12 of 76). There was no association between the ABCB1 genotype of the donor or recipient and P-glycoprotein expression at 3 mo after transplantation (Table S18). When the multivariate analysis for risk factors of IF/TA was repeated in this subgroup of patients with inclusion of the P-glycoprotein immunohistochemistry results at 3 mo as a covariate (n = 76), absence of apical P-glycoprotein expression in tubular epithelium was associated with higher IF/TA grade (OR 2.7 [1.0 to 7.5], P < 0.05), independent of donor age and the combined donor–recipient ABCB1 genotype (Figure S3 and Table S19). There was no association between P-glycoprotein staining and specific histologic evidence of calcineurin inhibitor nephrotoxicity like nodular arteriolar hyalinosis.
Renal Allograft Function and Survival.
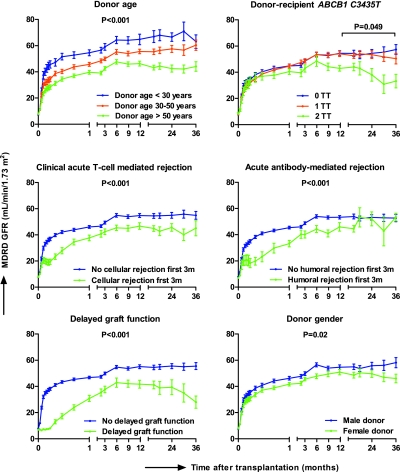
Delayed graft function defined as the need for dialysis in the first week after transplantation occurred in 42 patients (16.7%) and was independently associated with higher donor age (OR 1.04 [1.02 to 1.08] per year, P = 0.02), longer duration of cold ischemia (OR 1.1 [1.0 to 1.2] per hour, P = 0.02), and the number of prior transplantations (OR 2.1 [1.1 to 3.8], P = 0.02) (Table S20). The genotype of CYP3A4, CYP3A5, or ABCB1 of the donors and recipients was not associated with the risk for delayed graft function. Patients who underwent a biopsy within the first 2 wk after transplantation because of delayed graft function (n = 26 of 42 patients) had acute cellular rejection in 8 of 26 cases (30.8%), borderline acute cellular rejection in 4 of 26 (15.4%), pure antibody-mediated rejection in 2 of 26 cases (7.69%), and mixed antibody-mediated and acute cellular rejection in 8 of 26 cases (30.8%). Four of these biopsies did not have features of rejection (15.4%). Independent risk factors for worse graft function (lower MDRD glomerular filtration rate) in the first 3 yr after transplantation were higher donor age, female donor, longer duration of cold ischemia, acute T cell-mediated rejection in the first 3 mo after transplantation, and acute antibody-mediated rejection in the first 3 mo after transplantation (Figure 3 and Table S21). Independent risk factors for graft function after the first year were higher donor age, the combined donor–recipient ABCB1 TT status for the polymorphism at position 3435, diabetes mellitus at any time point after transplantation, and delayed graft function in the first week after transplantation. Absence of P-glycoprotein expression by immunohistochemistry on 3-mo biopsies was associated with decreased graft function in univariate analysis (P = 0.03), but this was no longer significant in the multivariate models.
Figure 3.
Determinants of renal allograft function in the first 3 yr after transplantation. Risk factors were selected using mixed-models repeated-measures analysis and backward elimination; P values less than 0.05 were required for inclusion in the final model (Table S21). n = 244, n = 242, n = 208, n = 173, n = 102, and n = 49 at 1, 3, 6, 12, 24, and 36 mo after transplantation, respectively. Results are given as mean ± SEM.
The appearance of the graft at implantation (glomerulosclerosis and IF/TA) was associated with graft function (MDRD glomerular filtration rate) up to 3 yr after transplantation. Also the presence of acute tubular necrosis in implantation biopsies correlated significantly with early graft function (up to 14 d after transplantation) but not with graft function at later time points (Table S22). The histologic appearance at 3 mo (glomerulosclerosis, IF/TA, and arteriolar hyalinosis) correlated significantly with graft function at all time points in the first 3 yr after transplantation (Table S22).
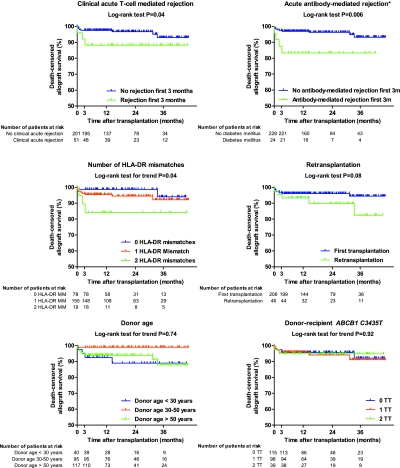
During follow-up, six patients died and 14 patients returned to dialysis, resulting in an actual death-censored graft survival of 96% at 1 yr, 95% at 2 yr, and 92% at 3 yr after transplantation (Table S23). Antibody-mediated and cellular rejection were the only determinants of early graft survival in our study cohort, next to the number of HLA-DR mismatches and retransplantation, which were shown to be the most important risk factors for both (sub)clinical acute cellular rejection and acute antibody-mediated rejection (Tables S8 through S11). Higher donor age or ABCB1 polymorphisms were not associated with graft survival up to 3 yr after transplantation (Figure 4).
Figure 4.
Determinants of renal allograft survival in the first 3 yr after transplantation, represented using Kaplan–Meier survival curves. No patients were lost to follow-up; patients were censored at the time of death with a functioning graft and when they completed the study before 3 yr after transplantation. P values were calculated using the log-rank test.
Discussion
This study describes the evolution of renal allograft histology in the first 3 yr years after transplantation and the risk factors for progressive renal allograft injury. Most interestingly, we demonstrate that the effects of higher donor age reach beyond the quality of the graft at implantation and continue to be important for histologic and functional evolution in the posttransplantation period, because higher donor age is associated with increased chronic tubulointerstitial damage independent of renal allograft histology at the time of transplantation. In addition and for the first time, our study provides prospective evidence that function (ABCB1 polymorphisms in both donors and recipients) and tubular epithelial cell expression of P-glycoprotein are important for the individual susceptibility to chronic tubulointerstitial injury of transplanted kidneys and graft function. This association of P-glycoprotein expression and function with chronic histologic damage of renal allografts is not mediated through changes in systemic tacrolimus exposure but likely through local renal accumulation of tacrolimus. Systemic exposure to tacrolimus, when maintained within relatively narrow targets, is not predictive of renal allograft histology. The effects of higher donor age and ABCB1 polymorphisms are reflected in graft functional evolution but not yet in graft survival, which is mainly determined by early acute T cell- and antibody-mediated rejection, in itself related to higher numbers of HLA-DR mismatches and prior transplantation, respectively. Longer-time follow-up is necessary to elucidate whether the observed progression of chronic histologic damage in protocol biopsies associates with late renal allograft loss.
Pre-existing structural damage of the donor kidney, which results from normal aging and medical conditions such as arterial hypertension and diabetes,23 is likely to accelerate secondary chronic injury processes in the transplanted kidney, through ischemic complications of vascular damage, increased hyperfiltration injury, sclerosis of remnant glomeruli, and increased susceptibility to other types of renal injury.13,24–26 However, the current longitudinal study demonstrates that the impact of higher donor age reaches beyond the quality of the donor kidney at implantation, because higher donor age remains the most important determinant of the histologic evolution in patients who received a histologically pristine kidney at implantation. This increased susceptibility of older donor kidneys to chronic injury is evident in the tubulointerstitial compartment but also in other renal compartments such as arterioles (arteriolar hyalinosis), small arteries (vascular intimal thickening), and glomeruli (global glomerulosclerosis). In the youngest donor kidneys, the absence of progression of peripheral arteriolar hyalinosis, which is considered a hallmark of calcineurin inhibitor nephrotoxicity,27 suggests that higher donor age is an important susceptibility (risk) factor for calcineurin inhibitor nephrotoxicity. This increased susceptibility of older kidneys to calcineurin inhibitor nephrotoxicity was suggested previously in patients treated with cyclosporine for other reasons28 and in an animal study that demonstrated that older animals with pre-existing age-related renal dysfunction developed significantly worse nephrotoxicity compared with younger animals.29 Finally, some indirect evidence is found also in the interaction between kidney age and calcineurin inhibitor cessation on the evolution of renal allograft function, with older donor kidneys having significantly less improvement of graft function after cyclosporine cessation compared with that of younger donor kidneys.30 Although this is no definite proof, this finding suggests that calcineurin inhibitors induce more irreversible damage to the kidneys from older donors compared with younger donors.
At least five coinciding phenomena can be held responsible for the increased susceptibility of older donor kidneys for posttransplantation injury and calcineurin inhibitor nephrotoxicity. First, it is expected that the normal aging process persists after organ transplantation and even could be accelerated.31,32 Despite sometimes normal structural appearance at implantation, renal cells from older donors will reach their cycling limit sooner than kidneys from younger donors and undergo specific molecular and morphologic changes associated with aging. Second, kidneys from older donors are more susceptible to typical transplantation-related injury such as ischemia–reperfusion injury33 and T cell-mediated rejection episodes.34 Third, the increased susceptibility of older kidneys to calcineurin inhibitor nephrotoxicity likely relates to age-related changes in renal autoregulation, with excessive vasoconstriction and anatomical changes of the preglomerular vasculature and subsequent ischemia of the glomeruli.35–37 It could be expected that the calcineurin-inhibitor-associated alterations in vasodilator and vasoconstrictory responses38 have more impact when these anatomical lesions and dysfunctions pre-exist. Fourth, senescence of renal tissue likely associates with impaired cellular repair mechanisms,39 which is particularly unfortunate for allografts from older donors in the context of increased susceptibility to histologic injury. Finally, age-related pre-existing or newly occurring vascular changes and tubulointerstitial damage (with interstitial microvascular injury) likely lead to increased susceptibility to downstream ischemic phenomena and further amplification of the effect of donor age on the histologic evolution of transplanted kidneys.
The current study demonstrates that both low P-glycoprotein expression in renal tubular epithelial cells and the TT variant at the 3435 position in ABCB1, which is associated with altered conformation and function of P-glycoprotein,40 are associated independently with increased risk for tubulointerstitial damage of renal allografts, without affecting systemic tacrolimus exposure. The mechanisms by which local renal expression of P-glycoprotein is regulated are not yet identified, but in agreement with previous studies40 we found no association between P-glycoprotein expression and ABCB1 genotype. P-glycoprotein is expressed at the apical side of tubular epithelial cells and is the main protein responsible for the excretion of tacrolimus and cyclosporine, although the renal excretion of these calcineurin inhibitors is only limited.14,41–43 It could be assumed that lower expression and decreased function of P-glycoprotein at the apical side of the proximal tubules leads to intracellular accumulation of tacrolimus and direct toxic effects on tubular cells, as was suggested previously for cyclosporine.15,18,44–47 In addition, it could be that decreased expression or altered function of P-glycoprotein plays a role in the apoptotic response to nephrotoxic agents and protects proximal tubular cells against apoptotic stress.48 Because cyclosporine use is associated with apoptotic mechanisms,44,49–51 defective P-glycoprotein activity or decreased expression could increase the direct nephrotoxic effects of calcineurin inhibition. This hypothesis would also be an explanation for the fact that there is an apparent discrepancy between the minor importance of the kidney in tacrolimus pharmacokinetics41,42 and the suggested contribution of local renal P-glycoprotein to calcineurin inhibitor nephrotoxicity.
Finally, we demonstrate that the effects of ABCB1 genotype are only evident when both donor and recipient are homozygous for the TT variant. We hypothesize that this is due to the local renal effects of P-glycoprotein function, because we could not demonstrate an effect of the ABCB1 genotype on tacrolimus pharmacokinetics or rejection incidence. The unexpected finding that also recipient polymorphisms impact on local graft injury susceptibility could be explained by the high prevalence of epithelial chimerism after kidney transplantation, with 88% of allografts having tubular epithelial cells in part from recipient origin,52 consistent with findings in other organs.53–56 However, although tubular chimerism was common after kidney transplantation in this study, only a few tubular cells were of recipient origin and a vast majority were donor in origin,52 although in our study the relative effects of donor and recipient ABCB1 genotype were similar. The reason for this apparent discrepancy is not clear and requires further investigation. It is of note that we could not confirm the previously described association between recipient ABCB1 genotype and acute rejection or delayed graft function in the current larger study.57 Whether this relates to the type of calcineurin inhibitor used deserves further study.
The current study has several clinical implications. First, although performing implantation biopsies is obviously of great importance for a first assessment of kidney quality, prediction, and even improvement of long-term outcome,56 they do not completely describe donor graft quality. Inclusion of clinical determinants such as donor age could further improve predictive accuracy. Second, it also should be emphasized that the effect of donor age reaches beyond the quality of the graft at implantation, and higher donor age also is associated with the progression of histologic lesions later after transplantation, independent of the histologic appearance at implantation. This is especially important for lesions such as new onset or progressive arteriolar hyalinosis, which is regarded as a marker of calcineurin inhibitor nephrotoxicity. This increased susceptibility to calcineurin inhibitor nephrotoxicity in older kidneys suggests that a calcineurin-inhibitor-free immunosuppressive protocol could be especially beneficial in kidneys from older donors. Third, parameters of tacrolimus exposure were associated neither with the evolution of chronic histologic damage nor with the risk for clinical or subclinical acute T cell- or antibody-mediated rejection. This does not mean that tacrolimus drug levels are irrelevant or that tacrolimus therapeutic drug monitoring is unnecessary but shows that the small variability in tacrolimus exposure (when maintained within a narrow target window) is not responsible for the interindividual variability in histologic evolution, at least not with the current immunosuppressive combination of tacrolimus, mycophenolate mofetil, and corticosteroids. Whether the findings are also applicable in patients treated with other immunosuppressive regimens remains to be demonstrated. Especially the type of immunosuppression used and the doses or target levels applied could be very relevant for the histologic evolution of kidney allografts. In this light, it is interesting to note the steep increase in the arteriolar hyalinosis score in the study by Nankivell et al.4 and the absence of evolution after the first posttransplant year in the current study. Finally, the association of epithelial P-glycoprotein expression and ABCB1 polymorphisms with the early histologic and functional evolution of kidney allografts suggests that screening for these polymorphisms and assessment of tubular epithelial P-glycoprotein expression could provide additional guidance about which patients will likely benefit most from a calcineurin-inhibitor-free or calcineurin-inhibitor-minimization immunosuppressive regimen.
Concise Methods
Histologic Evaluation
Slides containing 4 to 10 paraffin sections (2 μm) were stained with hematoxylin eosin, periodic acid–Schiff, and a silver methenamine staining method (Jones). An immunohistochemical C4d stain (monoclonal antibody, dilution 1:500, Quidel Corporation, Santa Clara, CA) was performed on frozen tissue. All protocol biopsies and indication biopsies included in this study were reviewed by one blinded pathologist (i.e., without knowledge of any clinical information or timing of the biopsy). The severity of histologic lesions (tubulitis, interstitial inflammation, intimal arteritis, glomerulitis, interstitial fibrosis [IF], tubular atrophy [TA], arteriolar hyalinosis, vascular intimal thickening, transplant glomerulopathy, and increase in mesangial matrix) was scored semiquantitatively according to the revised Banff criteria.21,22 In addition, the presence of microcalcifications and of pericapsular glomerular fibrosis and the number of sclerosed glomeruli were scored separately. Peritubular capillaritis was scored based on the maximal number of intraluminal leukocytes in a transverse section of a peritubular capillary, with a score from 0 to 3 (0 = no peritubular inflammatory changes; 1 = dilation of the peritubular capillaries with 3 to 4 luminal inflammatory cells; 2 = dilation of the peritubular capillaries with 5 to 10 luminal inflammatory cells; 3 = dilation of the peritubular capillaries with 10 luminal inflammatory cells). Acute antibody-mediated rejection was graded according to the histologic criteria of the revised Banff classification.21 Because we used a monoclonal antibody for C4d immunohistochemistry, C4d deposition in the peritubular capillaries was scored from 0 to 3 (0 = negative; 1 = <25%; 2 = 25 to 75%; and 3 = >75% of peritubular capillaries positive); C4d staining scores 2 and 3 were regarded as focal and diffuse positive, and score 1 was regarded as of unknown significance.21 Chronic antibody-mediated rejection was diagnosed when C4d score 2 or 3 coincided with transplant glomerulopathy. In addition, frozen sections of 3-mo biopsies of patients with at least 2 yr of follow-up were stained with a monoclonal antibody against P-glycoprotein (JSB-1 MmAb; dilution 1:10; Monosan, Sanbio B.V., Uden, The Netherlands). On the same day as the biopsy was performed, polyomavirus PCR was performed on peripheral blood. All biopsies of patients with concomitant polyomavirus viremia were stained with a monoclonal SV40 T Ag antiserum (Oncogene Research Products, Merck Chemicals Ltd., Nottingham, UK).
Tacrolimus Exposure
Tacrolimus pharmacokinetic profiles were obtained using a previously described limited sampling strategy in the first 4 h after dosing, with sampling at 0, 0.5,1, 1.5, 2, 3, and 4 h after dosing.58 Clinicians were blinded for the results of these extended pharmacokinetic measurements. Tacrolimus dose adjustments were based solely on C0 levels. Blood concentrations of tacrolimus were determined using the Tacrolimus II MEIA/IMx assay (Abbott Laboratories, Abbott Park, IL).
Pharmacogenetics
Single-nucleotide polymorphisms of ABCB1 (C3435T and G2677T/A), CYP3A4 (A-290G; CYP3A4*1 versus CYP3A4*1b), and CYP3A5 (G6986A; CYP3A5*1 versus CYP3A5*3) were determined using previously described methods (Table S24).
Statistical Analysis
The correlation between time after transplantation and the prevalence of different grades of histologic lesions was analyzed with the Cochran–Mantel–Haenszel statistic. Multiple (ordered) logistic regression analysis with backward selection was used to model the risk factors for the different dichotomous histologic patterns at implantation. To model the risk factors for the different histologic patterns by time after transplantation, the GENMOD procedure with a generalized-estimating-equations approach was used; final models were constructed after backward elimination. For assessing the clinical determinants of the time-dependent evolution of graft function and tacrolimus pharmacokinetics, mixed-models repeated-measures analysis was used. Correlations between continuous variables were assessed by Pearson correlation; with ordinal variables Spearman correlations were calculated. Odds ratios are presented with a 95% confidence interval. All P values are two-sided, and those <0.05 are considered to indicate statistical significance. Data analysis was performed using SAS software (SAS version 9.1; SAS Institute, Cary, NC).
Disclosures
None.
Acknowledgments
We acknowledge Prof. Bert Bammens, Dr. Kathleen Claes, Prof. Willy Coosemans, Dr. Liesbeth De Wever, Prof. Pieter Evenepoel, Prof. Diethard Monbaliu, Prof. Raymond Oyen, and Prof. Jacques Pirenne for their enthusiastic collaboration in the protocol biopsy project. We thank Marina Dubois, Albert Herelixka, Isabelle Laenen, Anja Swinnen, André Van Esch, and Helga Wielandt for their excellent support. We are indebted to Marc Dekens, Henriette de Loor, and Eveline Vanhalewyck from the Laboratory of Nephrology, to Prof. Marie-Paule Emonds and the technical staff of the HLA Laboratory, to Prof. Severine Vermeire and Prof. Kristin Verbeke and the Laboratory of Gastroenterology, and to Dr. Sara Vander Borght of the Laboratory of Morphology and Molecular Pathology. We thank the centers of the Leuven Collaborative Group for Renal Transplantation, the clinicians and surgeons, nursing staff, and of course the patients. M.N. and H.D.J. are funded by the Research Foundation—Flanders (FWO). M.N., E.L., and D.R.J.K. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented as an abstract at the 22nd International Congress of The Transplantation Society (August 10 through August 14, 2008, Sydney, Australia) and at the 9th American Transplant Congress (May 30 through June 3, 2009, Boston, MA).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Allograft Biopsies: Studying Them for All They're Worth,” on pages 2282–2284.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Chapman JR, O'Connell PJ, Nankivell BJ: Chronic renal allograft dysfunction. J Am Soc Nephrol 16: 3015–3026, 2005 [DOI] [PubMed] [Google Scholar]
- 2.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Am H, Gloor JM, Cosio FG: Identifying specific causes of kidney allograft loss. Am J Transplant 9: 527–535, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Yilmaz S, Tomlanovich S, Mathew T, Taskinen E, Paavonen T, Navarro M, Ramos E, Hooftman L, Häyry P: Protocol core needle biopsy and histologic Chronic Allograft Damage Index (CADI) as surrogate end point for long-term graft survival in multicenter studies. J Am Soc Nephrol 14: 773–779, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Nankivell B, Richard J, Borrows R, Fung CL-S, O'Connell PJ, Allen R, Chapman JR: The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Ojo AO, Hanson JA, Meier-Kriesche H, Okechukwu CN, Wolfe RA, Leichtman AB, Agodoa LY, Kaplan B, Port FK: Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol 12: 589–597, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Remuzzi G, Cravedi P, Perna A, Dimitrov BD, Turturro M, Locatelli G, Rigotti P, Baldan N, Beatini M, Valente U, Scalamogna M, Ruggenenti P: Long-term outcome of renal transplantation from older donors. N Engl J Med 354: 343–352, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Halloran PF: Immunosuppressive drugs for kidney transplantation. N Engl J Med 351: 2715–2729, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Andreoni KA, Brayman KL, Guidinger MK, Sommers CM, Sung RS: Kidney and pancreas transplantation in the United States, 1996–2005. Am J Transplant 7: 1359–1375, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Moreso F, Serón D, Carrera M, Gil-Vernet S, Cruzado J, Hueso M, Fulladosa X, Ramos R, Ibernon M, Castelao A, Grinyó JM: Baseline immunosuppression is associated with histological findings in early protocol biopsies. Transplantation 78: 1064–1068, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Nankivell BJ, Borrows RJ, Fung C L-S, O'Connell PJ, Allen RD, Chapman JR: Natural history, risk factors, and impact of subclinical rejection in kidney transplantation. Transplantation 78: 242–249, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, Margreiter R, Hugo C, Grinyo JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF: Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Cornell LD, Colvin RB: Chronic allograft nephropathy. Curr Opin Nephrol Hypertens 14: 229–234, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Nankivell BJ, Chapman JR: Chronic allograft nephropathy: Current concepts and future directions. Transplantation 81: 643–654, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Saeki T, Ueda K, Tanigawara Y, Hori R, Komano T: Human P-glycoprotein transports cyclosporin A and FK506. J Biol Chem 268: 6077–6080, 1993 [PubMed] [Google Scholar]
- 15.del Moral RG, Andujar M, Ramirez C, Gomez-Morales M, Masseroli M, Aguilar M, Olmo A, Arrebola F, Guillen M, Garcia-Chicano MJ, Nogales FF, O'Valle F: Chronic cyclosporin A nephrotoxicity, P-glycoprotein overexpression, and relationships with intrarenal angiotensin II deposits. Am J Pathol 151: 1705–1714, 1997 [PMC free article] [PubMed] [Google Scholar]
- 16.Koziolek MJ, Riess R, Geiger H, Thevenod F, Hauser IA: Expression of multidrug resistance P-glycoprotein in kidney allografts from cyclosporine A-treated patients. Kidney Int 60: 156–166, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Joy MS, Nickeleit V, Hogan SL, Thompson BD, Finn WF: Calcineurin inhibitor-induced nephrotoxicity and renal expression of P-glycoprotein. Pharmacotherapy 25: 779–789, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Anglicheau D, Pallet N, Rabant M, Marquet P, Cassinat B, Meria P, Beaune P, Legendre C, Thervet E: Role of P-glycoprotein in cyclosporine cytotoxicity in the cyclosporine–sirolimus interaction. Kidney Int 70: 1019–1025, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hebert MF, Dowling AL, Gierwatowski C, Lin YS, Edwards KL, Davis CL, Marsh CL, Schuetz EG, Thummel KE: Association between ABCB1 (multidrug resistance transporter) genotype and post-liver transplantation renal dysfunction in patients receiving calcineurin inhibitors. Pharmacogenetics 13: 661–674, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Hauser IA, Schaeffeler E, Gauer S, Scheuermann EH, Wegner B, Gossmann J, Ackermann H, Seidl C, Hocher B, Zanger UM, Geiger H, Eichelbaum M, Schwab M: ABCB1 genotype of the donor but not of the recipient is a major risk factor for cyclosporine-related nephrotoxicity after renal transplantation. J Am Soc Nephrol 16: 1501–1511, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Solez K, Axelsen R, Benediktsson H, Burdick J, Cohen A, Colvin R, Croker B, Droz D, Dunnill MS, Halloran PF, Hayry P, Jennette JC, Keown P, Marcussen N, Mihatsch MJ, Morozumi K, Myers B, Nast C, Olsen S, Racusen L, Ramos E, Seymour R, Sachs D, Salomon DR, Sanfilippo F, Verani H, von Willebrand E, Yamaguchi Y: International standardization of criteria for the histologic diagnosis of renal allograft rejection: The Banff working classification of kidney transplant pathology. Kidney Int 44: 411–422, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Epstein M: Aging and the kidney. J Am Soc Nephrol 7: 1106–1122, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Nenov VD, Taal MW, Sakharova OV, Brenner BM: Multi-hit nature of chronic renal disease. Curr Opin Nephrol Hypertens 9: 85–97, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM: Hyperfiltration in remnant nephrons: A potentially adverse response to renal ablation. J Am Soc Nephrol 12: 1315–1325, 2001. 11373357 [Google Scholar]
- 26.Alperovich G, Maldonado R, Moreso F, Fulladosa X, Grinyo JM, Seron D: Glomerular enlargement assessed by paired donor and early protocol renal allograft biopsies. Am J Transplant 4: 650–654, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Young BA, Burdmann EA, Johnson RJ, Andoh T, Bennett WM, Couser WG, Alpers CE: Cyclosporine A induced arteriolopathy in a rat model of chronic cyclosporine nephropathy. Kidney Int 48: 431–438, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Feutren G, Mihatsch MJ: Risk factors for cyclosporine-induced nephropathy in patients with autoimmune diseases. International Kidney Biopsy Registry of Cyclosporine in Autoimmune Diseases. N Engl J Med 326: 1654–1660, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Bennett WM, Lindsley J, Buss WC: The effects of age and dosage route on experimental cyclosporine nephrotoxicity. Transplantation 51: 730–731, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Legendre C, Brault Y, Morales JM, Oberbauer R, Altieri P, Riad H, Mahony J, Messina M, Pussell B, Martinez JG, Lelong M, Burke JT, Neylan JF: Factors influencing glomerular filtration rate in renal transplantation after cyclosporine withdrawal using sirolimus-based therapy: A multivariate analysis of results at five years. Clin Transplant 21: 330–336, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Halloran PF, Melk A, Barth C: Rethinking chronic allograft nephropathy: The concept of accelerated senescence. J Am Soc Nephrol 10: 167–181, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Jennings P, Koppelstaetter C, Aydin S, Abberger T, Wolf AM, Mayer G, Pfaller W: Cyclosporine A induces senescence in renal tubular epithelial cells. Am J Physiol Renal Physiol 293: F831–F838, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Moreso F, Seron D, Gil-Vernet S, Riera L, Fulladosa X, Ramos R, Alsina J, Grinyo JM: Donor age and delayed graft function as predictors of renal allograft survival in rejection-free patients. Nephrol Dial Transplant 14: 930–935, 1999 [DOI] [PubMed] [Google Scholar]
- 34.de Fijter JW, Mallat M, Doxiadis I, Ringers J, Rosendaaal F, Claas FH, Paul LC: Increased immunogenicity and cause of graft loss of old donor kidneys. J Am Soc Nephrol 12: 1538–1546, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Jung FF, Kennefick TM, Ingelfinger JR, Vora JP, Anderson S: Down-regulation of the intrarenal renin-angiotensin system in the aging rat. J Am Soc Nephrol 5: 1573–1580, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Luke RG: Hypertensive nephrosclerosis: Pathogenesis and prevalence. Essential hypertension is an important cause of end-stage renal disease. Nephrol Dial Transplant 14: 2271–2278, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Long DA, Mu W, Price KL, Johnson RJ: Blood vessels and the aging kidney. Nephron Exp Nephrol 101: e95–e99, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Burdmann EA, Andoh TF, Yu L, Bennett WM: Cyclosporine nephrotoxicity. Semin Nephrol 23: 465–476, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Schmitt R, Cantley LG: The impact of aging on kidney repair. Am J Physiol Renal Physiol 294: F1265–F1272, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM: A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 315: 525–528, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Fahr A: Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet 24: 472–495, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Staatz CE, Tett SE: Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet 43: 623–653, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Ernest S, Rajaraman S, Megyesi J, Bello-Reuss EN: Expression of MDR1 (multidrug resistance) gene and its protein in normal human kidney. Nephron 77: 284–289, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Shihab FS, Andoh TF, Tanner AM, Yi H, Bennett WM: Expression of apoptosis regulatory genes in chronic cyclosporine nephrotoxicity favors apoptosis. Kidney Int 56: 2147–2159, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Ling H, Li X, Jha S, Wang W, Karetskaya L, Pratt B, Ledbetter S: Therapeutic role of TGF-β-neutralizing antibody in mouse cyclosporin A nephropathy: Morphologic improvement associated with functional preservation. J Am Soc Nephrol 14: 377–388, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Napoli KL, Wang ME, Stepkowski SM, Kahan BD: Relative tissue distributions of cyclosporine and sirolimus after concomitant peroral administration to the rat: Evidence for pharmacokinetic interactions. Ther Drug Monit 20: 123–133, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Podder H, Stepkowski SM, Napoli KL, Clark J, Verani RR, Chou TC, Kahan BD: Pharmacokinetic interactions augment toxicities of sirolimus/cyclosporine combinations. J Am Soc Nephrol 12: 1059–1071, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Thevenod F, Friedmann JM, Katsen AD, Hauser IA: Up-regulation of multidrug resistance P-glycoprotein via nuclear factor-κB activation protects kidney proximal tubule cells from cadmium- and reactive oxygen species-induced apoptosis. J Biol Chem 275: 1887–1896, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Thomas SE, Andoh TF, Pichler RH, Shankland SJ, Couser WG, Bennett WM, Johnson RJ: Accelerated apoptosis characterizes cyclosporine-associated interstitial fibrosis. Kidney Int 53: 897–908, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Longoni B, Boschi E, Demontis GC, Ratto GM, Mosca F: Apoptosis and adaptive responses to oxidative stress in human endothelial cells exposed to cyclosporin A correlate with BCL-2 expression levels. FASEB J 15: 731–740, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Yang CW, Faulkner GR, Wahba IM, Christianson TA, Bagby GC, Jin DC, Abboud HE, Andoh TF, Bennett WM: Expression of apoptosis-related genes in chronic cyclosporine nephrotoxicity in mice. Am J Transplant 2: 391–399, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Mengel M, Jonigk D, Marwedel M, Kleeberger W, Bredt M, Bock O, Lehmann U, Gwinner W, Haller H, Kreipe H: Tubular chimerism occurs regularly in renal allografts and is not correlated to outcome. J Am Soc Nephrol 15: 978–986, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Kleeberger W, Rothamel T, Glockner S, Flemming P, Lehmann U, Kreipe H: High frequency of epithelial chimerism in liver transplants demonstrated by microdissection and STR-analysis. Hepatology 35: 110–116, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Korbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, Champlin RE, Estrov Z: Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med 346: 738–746, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Kleeberger W, Versmold A, Rothamel T, Glockner S, Bredt M, Haverich A, Lehmann U, Kreipe H: Increased chimerism of bronchial and alveolar epithelium in human lung allografts undergoing chronic injury. Am J Pathol 162: 1487–1494, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suratt BT, Cool CD, Serls AE, Chen L, Varella-Garcia M, Shpall EJ, Brown KK, Worthen GS: Human pulmonary chimerism after hematopoietic stem cell transplantation. Am J Respir Crit Care Med 168: 318–322, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Cattaneo D, Ruggenenti P, Baldelli S, Motterlini N, Gotti E, Sandrini S, Salvadori M, Segoloni G, Rigotti P, Donati D, Perico N, Remuzzi G: ABCB1 genotypes predict cyclosporine-related adverse events and kidney allograft outcome. J Am Soc Nephrol 20: 1404–1415, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuypers DRJ, Claes K, Evenepoel P, Maes B, Coosemans W, Pirenne J, Vanrenterghem Y: Time-related clinical determinants of long-term tacrolimus pharmacokinetics in combination therapy with mycophenolic acid and corticosteroids: A prospective study in one hundred de novo renal transplant recipients. Clin Pharmacokinet 43: 741–762, 2004 [DOI] [PubMed] [Google Scholar]