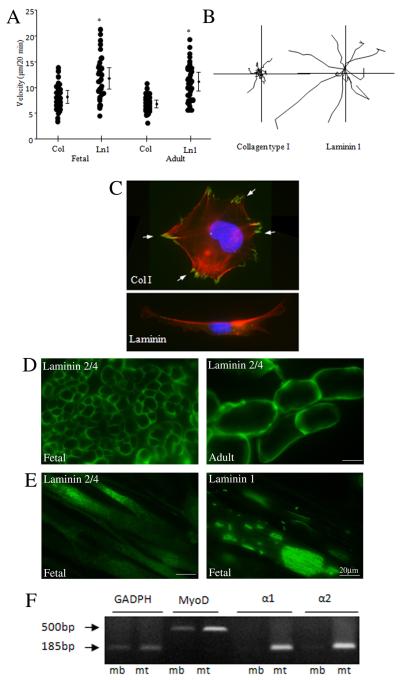
Figure 3. Laminin, a muscle stem cell niche component, promotes migration of human myoblasts.
(A) Cell motility of fetal and adult muscle cells was measured after seeding cells on type I collagen and laminin-1 coated substrates by video time-lapse microscopy. Asterisk* P<0.001 denotes significant differences between migration on collagen and laminin. (B) Individual cell tracks are shown for each substrate for fetal cells. (C) Focal adhesion formation on type I collagen and laminin-1 substrates. Fetal cells were plated on coverslips coated with type I collagen or laminin-1. After 2 h, cells were fixed, stained for paxillin and philloidin (polymerized actin), and examined by immunofluorescence microscopy. The localization of paxillin in focal adhesion on collagen is indicated by arrows (D) Immunostaining of fetal and adult muscle sections using antibodies to laminin α2 chain shows expression on the surface of individual muscle fibers. (E) Immunostaining for laminin α2 chain and laminin α1 chain showed expression of these isoforms by differentiated human myotubes in culture with a unique surface localization pattern for laminin α1. (F) Semiquantitative RT-PCR detected mRNA for α1 and α2 chains of laminin in fetal myotubes (mt) but not in myoblast (mb) cultures. MyoD mRNA expression, included as a positive control, was elevated in differentiated cultures.