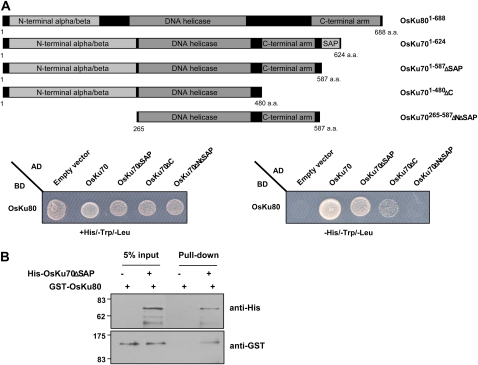
Figure 2.
Interactions between OsKu70 and OsKu80. A, Yeast two-hybrid assay. Full-length OsKu70 and its deletion mutants (OsKu701–587-ΔSAP, OsKu701–480-ΔC, and OsKu70265–587-ΔNΔSAP) were cloned into pGADT7, while full-length OsKu80 was cloned into pGBKT7. Combinations of the indicated plasmids were cotransformed into yeast AH109 cells. Transformed yeast cells were plated onto SD/+His/−Trp/−Leu or SD/−His/−Trp/−Leu medium containing 10 mm 3-aminotriazole and grown for 4 d at 28°C. The capacity of the yeast cells to grow in the absence of His was used as a readout for the interaction between OsKu70 and OsKu80. a.a., Amino acids; AD, activation domain; BD, binding domain. B, In vitro pull-down assay. The bacterially expressed (His)6-OsKu701–587-ΔSAP fusion protein was incubated with GST-OsKu80 in the presence of Ni-NTA resin. After extensive washing with immunoprecipitation buffer, the bound proteins were eluted from the affinity resin by boiling with SDS sample buffer. The eluted proteins were resolved by 10% SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and subsequently immunoblotted with anti-His and anti-GST antibodies. [See online article for color version of this figure.]