Abstract
Chitosan microspheres as drug delivery system have attained importance and attracted the attention of researchers in last few years. This study was aimed toward the elucidation of the effect of viscosity of external oil phase on the properties of microspheres prepared by emulsification method. Chitosan microspheres were prepared utilizing oil phase of different viscosity viz. castor oil, heavy liquid paraffin, light liquid paraffin and mixture of light paraffin, and petroleum ether (1:1 v/v ratio). Microspheres prepared in highly viscous castor oil exhibited an average size of 11.52 ± 0.57 µm with a percentage drug entrapment of 43.12 ± 2.14. On the other hand, very small microspheres of 3.15 ± 0.04 µm and 68.87 ± 1.03% drug entrapment were obtained when mixture of liquid paraffin and petroleum ether was utilized as oil phase. Effect of viscosity on percent mucoadhesion, percent drug entrapment, zeta potential, percent process yield, etc. of microspheres has been observed. In vitro drug release in phosphate buffer pH 7.4 was determined for different batch of microspheres. The results revealed a difference in the drug release pattern of the different microspheres prepared as a function of viscosity of different oil phase. Use of low viscose oil resulted in the formulation of spherical and small size microspheres. This work was a part of our ongoing thrust and project to develop microparticulate drug delivery system.
Key words: chitosan, emulsification, microspheres, release study, viscosity
INTRODUCTION
Microspheres have been explored extensively for their use in the field of drug delivery, and various polymers have been utilized for the formulation of the microspheres which in turn have been assessed for different purposes. Last few years have witnessed dosage forms that can precisely control the release rates and target drugs to a definite body site or organ. These have played a pivotal role and provided a major thrust in the development of the novel drug delivery field. Microspheres are potential drug delivery carrier systems in the segment of novel drug delivery (1–3) and are prepared using assorted polymers (4). Chitosan which is deacetylated derivative of (-4)2-acetamido-2-deoxy-b-d-glucose or chitin has been extensively explored for its various biomedical and pharmaceutical applications (5–13). Properties such as biodegradability, low toxicity, and good biocompatibility make it suitable for use in drug delivery and biomedical field (14, 15). As a drug carrier, chitosan has been investigated for the sustained delivery of many oral formulations and parenteral formulations (16–22). There are various methods for the preparation of the chitosan microspheres including the emulsification method which remains the common method on laboratory scale. A number of reports and studies have been published regarding the effect of cross-linking agent and other variables on the quality and properties of the chitosan microspheres but almost no studies have been reported so far with respect to the effect of viscosity of oil phase on the formulation and characteristics of the resultant microspheres which is also an important aspect and variable in the process of development of microspheres. This study focuses and is aimed toward deciphering the role of viscosity of various oil phase and as to how it tends to effect the characteristics of the resultant chitosan microspheres which have been formulated by emulsification method utilizing oil phase of different viscosity. Different properties of resulting microspheres have been studied and reported, correlating them with the effect produced on them by the viscosity of the different oil phases. Fourth generation cephalosporin, ceftriaxone sodium, was taken as model drug to study drug entrapment and in vitro release pattern of the drug from different microspheres prepared.
MATERIALS AND METHODS
Chitosan (purified, viscosity grade 50) obtained from Central Institute of Fisheries and Technology, Cochin India was used without further purification. Span 85 and glutaraldehyde (biological grade, 25% v/v aqueous solution) were from Sigma chemical, USA. Castor oil was from Williams Lab Mumbai, India. Heavy liquid paraffin and light liquid paraffin, petroleum ether, acetone, acetic acid, and other solvents were from Loba Chemie Pvt Ltd, Mumbai. India and were utilized as received. Deionized Millipore water was used throughout the study. Ceftriaxone sodium was a kind gift from Solisto Pharma, Sagar (M.P), India.
Rheological Measurements
Viscosity of various oil phases utilized in the study viz. castor oil, heavy liquid paraffin, light liquid paraffin and mixture of petroleum ether, and light liquid paraffin (1:1 v/v ratio) was measured using Brookfield viscometer (Model Dv-I+ viscometer, Brookfield Engineering Lab, Inc. USA). Low viscosity (LV) spindle was utilized and operated at 60 rpm. All the measurements were done at room temperature (Table I).
Table I.
Viscosity of Different Oil Phases
| Serial number | Oil phase | Viscosity (cs) |
|---|---|---|
| 1 | Castor oil | 1,093 ± 14.81 |
| 2 | Heavy liquid paraffin | 521 ± 9.12 |
| 3 | Light liquid paraffin | 80 ± 2.33 |
| 4 | Mixture of petroleum ether and light liquid paraffin (1:1 v/v ratio) | 72 ± 1.98 |
Data shown is average of three determinations. Values shown are mean ± SD, where n = 3
cs centipoise
Preparation of Microspheres
Microspheres were prepared by slight modification of emulsification method as described by Thanoo et al. (23). Glutaraldehyde has been widely used as cross-linking agent previously (24, 25). Microspheres were prepared by taking four different oil phase viz. castor oil, heavy liquid paraffin, light liquid paraffin, and a 1:1 mixture of petroleum ether and light liquid paraffin. Briefly, the drug was already dissolved in 4% acetic acid solution which was maintained at pH 3.5 with the help of acetate buffer pH 4.0, and then, chitosan was dissolved in the drug solution to produce 2% w/v concentration of the polymer. The drug to polymer ratio was kept 1:2. This aqueous phase (5 ml) was added dropwise (at a rate of 2 ml/min) to the beaker containing 50 ml of oil phase and 0.5% w/v Span 85 as emulsifying agent (previously mixed through stirring) under constant stirring. Stirring was carried out at 3,000 rpm utilizing a three blade mechanical stirrer (Remi Instruments, Mumbai). Fifteen percent glutaraldehyde was added after 20 min. The stirring was continued for 3 h. The oil phase containing microspheres was centrifuged at 1,000×g for 1 min. The supernatant oil was decanted, and the microspheres at the bottom were then washed three times with petroleum ether by centrifugation to remove the oil. They were then washed with water to remove traces of glutaraldehyde, then they were washed with acetone, and then, they were freeze-dried in a freeze drier (Heto Hilton, Germany).
Electron Microscopy
Shape and surface morphology of the different microspheres formed was investigated through Scanning Electron Microscope (Leo 435 VP, Oxford Instruments, England). Briefly, microspheres were sprinkled on double-sided tape, sputter coated with gold, and were examined under the microscope.
Particle Size
Microspheres were sized using a Malvern mastersizer (model Nano ZS-90, Malvern Instruments Private Ltd. UK). Microspheres were suspended in phosphate-buffered saline (PBS) pH 7.4. Two milliliters of this suspension was placed in the cuvette of the instrument, and the volume mean diameter and distribution of particle size were measured.
Zeta Potential
Zeta potentials were measured by electrophoresis performed on a Malvern Zetasizer (model Nano ZS-90, Malvern Instruments Private Ltd. UK). PBS pH 7.4 was used as the medium to suspend the microspheres for the measurement of the zeta potential.
Percent Mucoadhesion
The mucoadhesive properties of the different microspheres were evaluated by in vitro wash-off test as reported by Lehr et al. (26). A 1 × 1-cm piece of rat stomach was tied onto a glass slide using thread. Microspheres were spread (~50) onto the wet, rinsed tissue specimen, and the prepared slide was hung onto one of the grooves of a United States Pharmacopeia tablet disintegrating test apparatus. The disintegrating test apparatus was operated such that the tissue specimen was given regular up and down movements in a beaker containing the phosphate saline buffer pH 7.4. At the end of 1 h, the number of microspheres still adhering onto the tissue was counted.
Drug Content and Entrapment Efficiency
Microspheres were crushed in a glass mortar and pestle. The powered microspheres were suspended in 10 ml of phosphate buffer (pH 7.4). After 24 h, the solution was filtered, and the filtrate was analysed for the drug content through ultraviolet (UV) spectrophotometer (Shimadzu-1601, Kyoto, Japan) at 241.5 nm. The drug entrapment efficiency was calculated using the following formula:
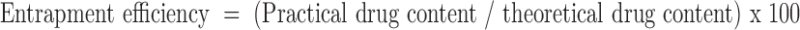 |
In Vitro Drug Release Study
In vitro drug release studies were performed by suspending 50 mg of microspheres in 2 ml of PBS (pH 7.4). This suspension was then filled in a 3 × 1-cm dialysis bag (12,000–14,000 Da MWCO, Sigma, Germany) which was previously hydrated by keeping it in deionized ultrapure water for 15 min, and this was placed in release media (50 ml of PBS pH 7.4) which was stirred at 50 rpm. The temperature of the medium was kept at 37 ± 2 C. Aliquots (1 ml) were withdrawn at predetermined intervals of time, and volume was made up with an equal amount of phosphate saline buffer pH 7.4. Similar procedure was carried out for all the samples. The aliquots were analyzed for the drug content at 241.5 nm using UV spectrophotometer (Shimadzu-1601, Kyoto, Japan). The experiment was repeated with n = 3, and values are average of three determinations (mean ± standard deviation (SD)).
RESULTS
Different batches of microspheres viz. MC, MH, ML, and MPL were prepared using different oil phase, castor oil, heavy liquid paraffin, light liquid paraffin, and 1:1 v/v ratio of Light liquid paraffin and petroleum ether, respectively. The effect of viscosity of oil phase (dispersion media) on the characteristics of microspheres was evaluated.
Effect on Size and Shape of the Microspheres
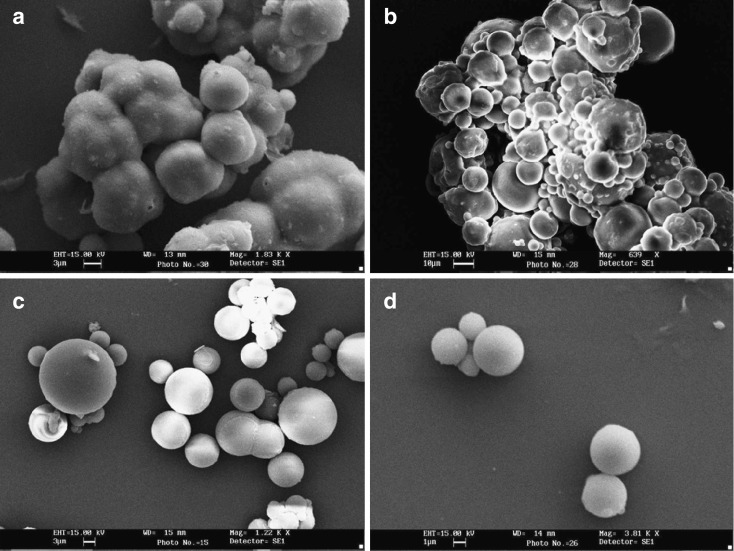
The mean size of the microspheres MC prepared using castor oil was 11.52 ± 0.57 µm whereas it was 9.62 ± 0.43 µm for MH (prepared in heavy paraffin), 5.62 ± 0.14 µm for ML (prepared in light liquid paraffin), and 3.15 ± 0.04 µm for MPL (mixture of light liquid paraffin and petroleum ether). The results have been tabulated in Table II. Figure 1 depicts the SEM photomicrographs of the different microspheres prepared in different oil phase.
Table II.
Physicochemical Characteristics of Different Chitosan Microspheres
| Serial number | Formulation code | Preparation medium | Size (µm) | PDI | Zeta potential (mv) | Percentage entrapment efficiency | Percent mucoadhesivity | Characteristics | Percent process yield |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MC | Castor oil | 11.52 ± 0.57 | 1.01 ± 0.046 | 1.13 ± 0.04 | 43.12 ± 2.14 | 58 | Heavily clumped | 47 |
| 2 | MH | Heavy liquid paraffin | 9.62 ± 0.43 | 0.88 ± 0.035 | 2.47 ± 0.08 | 52.61 ± 1.35 | 66 | Large aggregates | 53 |
| 3 | ML | Light liquid paraffin | 5.62 ± 0.14 | 0.41 ± 0.012 | 2.86 ± 0.05 | 65.32 ± 1.21 | 72 | Loosely aggregated and redispersible | 70 |
| 4 | MPL | 1:1 mixture of petroleum ether and light liquid paraffin | 3.15 ± 0.04 | 0.20 ± 0.02 | 3.05 ± 0.04 | 68.87 ± 1.03 | 75 | Free flowing | 73 |
Values shown are mean ± SD, where n = 3
PDI polydispersion index
Fig. 1.
In vitro drug release from different microsphere formulations prepared utilizing various oil phases. Series 1 drug release from MC, Series 2 drug release from MH, Series 3 drug release from ML, and Series 4 drug release from MPL
Effect on Mucoadhesivity
The mucoadhesive property of the different microspheres was determined as described earlier. It was found that percentage mucoadhesivity of formulation MC (prepared in castor oil) was 49% whereas it was 65% for formulation MH. It was 72% for formulation ML and 75%for formulation MPL (prepared in a mixture of light liquid paraffin and petroleum ether).
Effect on Zeta Potential
Zeta potential is indicative of the charge present on the surface of the particulate system and depends upon the migration of the particles in an electrostatic field. Positively charged free amine groups present on the surface of the chitosan microspheres contribute to the zeta potential of the formulation. It was found that zeta potential was 1.13 ± 0.04 mv for microspheres prepared in castor oil (formulation MC), 2.47 ± 0.08 mv for those prepared in heavy liquid paraffin, and 2.86 ± 0.05 mv for microspheres prepared in light liquid paraffin whereas it was 3.05 ± 0.04 mv for microspheres prepared in 1:1 v/v mixture of light paraffin and petroleum ether. The values have been tabulated in Table II.
Effect on Drug Entrapment Efficiency
Entrapment efficiency was calculated according to the method already described in the previous section of this paper. It was found to be 43.12 ± 2.14% for MC, 52.61 ± 1.35% for MH, and 65.32 ± 1.21% for ML whereas it was 68.87 ± 1.03% for MPL. The higher drug entrapment efficiency in the case of formulation MPL could be due to the uniform-sized microspheres as compared to the clumped microspheres in case of MC.
Effect on In Vitro Drug Release
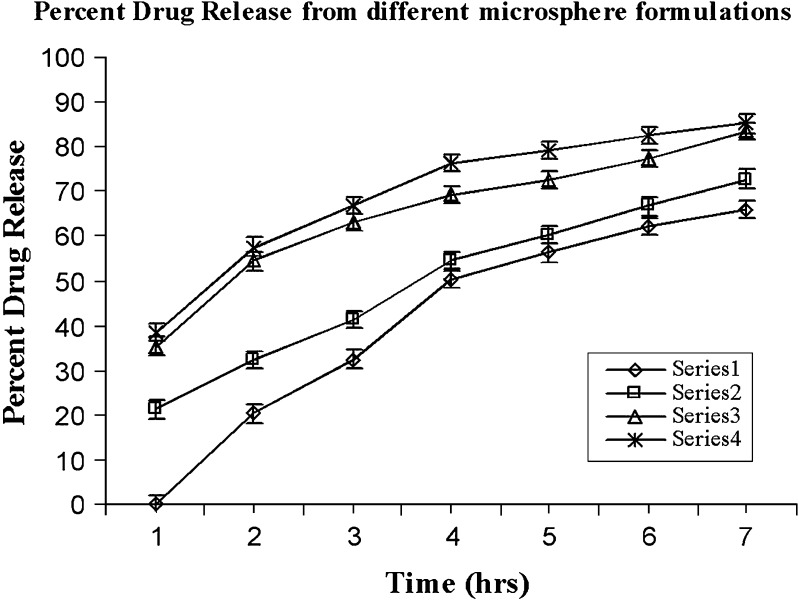
Percent drug release was calculated at different time intervals, i.e., 1, 2, 3, 4, and 7 h. It was noticed that there was no drug release after an interval of 1 h from formulation MC (prepared in castor oil). After second hour and onward, a very slow and uneven drug release was noticed which may be a result of constant agitation of dissolution media by which a part of the oily layer may have been eroded thus resulting in drug diffusion and drug release. Drug (65.97 ± 2.61%) was released at the end of 7 h. For formulation MH prepared in heavy paraffin, the drug release was slower in the beginning, which gradually increased, resulting in 72.69 ± 2.18% drug release at the end of 7 h. Drug (83.45 ± 2.08%) was released from formulation ML at the end of seventh hour whereas 85.12 ± 1.27% of drug was released from formulation MPL. This has been depicted graphically in Fig. 2.
Fig. 2.
Scanning electron micrographs of chitosan microspheres prepared a in castor oil, b in heavy liquid paraffin, c in light liquid paraffin, and d in mixture of light paraffin and petroleum ether (1:1 v/v ratio)
DISCUSSION
Shearing force plays a pivotal role in the formulation of microspheres through emulsification method. It was noticed that microspheres prepared in castor oil were large, uneven, and clumped whereas the microspheres prepared in heavy liquid paraffin (formulation MH) were large, nearly spherical, and existed in aggregates. On the other hand, the microspheres prepared in light liquid paraffin were smaller with very less aggregates being observed. The microspheres prepared in the 1:1 v/v light liquid paraffin and petroleum ether (formulation MPL) existed in monodisperse form, and they were free flowing along with being uniformly sized and smaller size. This phenomenon can be attributed to the relation between shear stress and coefficient of viscosity of different oil phase which can be explained by the formula:
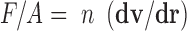 |
where F/A = force per unit surface area, n = coefficient of viscosity, and dv/dr = velocity gradient (27). Newton's law states that with the increase in the viscosity of a liquid, greater the force is required to produce a given rate of shear. In the present study, the force is applied by the rotation of the propeller blade where the force is directly proportional to the rotational speed. Hence, in the study, we realized that when rotations per minute of the stirrer are kept constant, so will the force per unit area be constant in the preparation of the microspheres in different oil phase. Thus, critically assessing, if we increase the viscosity of the oil phase keeping the rotations per minute of the stirrer constant, the shear stress will decrease and also be nonuniform. Hence, it is due to this reason that microspheres formed in highly viscous castor oil are larger in size and uneven in shape, which may be due to formation of larger droplets of water-in-oil (w/o) emulsion formed (Fig. 1a, formulation MC). Again, there is also a tendency of these larger droplets of w/o emulsion to coalesce due to reduced and uneven shear. This is concordant with the report of Wang et al. in where broader size distribution has been mentioned on increase of viscosity of oil phase (28). Moreover, these larger droplets undergo cross-linking at the time of cross-linking, which could be a possible reason for the formation of clumped microspheres. In another case, when heavy liquid paraffin was taken (Fig. 1b, formulation MH), the clumps seemed to reduce, the microspheres existed in large aggregates, and the size was larger as well. Also, they lacked uniformity. This could be due to uneven shear stress, whereas the batch of microspheres prepared using light liquid paraffin (Fig. 1c, formulation ML), which is less viscous than castor oil and heavy liquid paraffin, resulted in smaller and uniform microspheres, which could be due to an even and increased shear stress due to lowering of viscosity of the oil phase. It was observed that considerable portion of the microspheres prepared was free flowing with rest of the part existing as loose aggregates. On the other hand, free flowing, even, and uniform microspheres were obtained when we utilized a 1:1 ratio of light liquid paraffin and petroleum ether as oil phase (Fig. 1d, formulation MPL). This could be due to an increased and high shear stress, which is a result of lower viscosity of the mixture. Again, petroleum ether allowed the greater diffusion of the cross-linking agent added, and hence, even and efficient cross-linking of the very small droplets of the w/o emulsion formed would have been occurred. Moreover, the even and uniform shear stress resulted in the formation of small and uniform microspheres.
Marked effect on the mucoadhesive nature of the microspheres was observed, and as evident from the results, lower values of mucoadhesivity for formulation MC and MH could be due to the layer of oil on the surface of microspheres which could have considerably lowered down the mucoadhesiveness which in turn could be a result of highly viscous oil phase which was not removed from the surface even after several washings from petroleum ether. The greater values in case of formulation MP and MPL could be due to removal of the oil layer from the surface of the microspheres thereby exposing the free amine groups and hence, enhanced mucoadhesivity.
An interesting effect was noticed in the case of zeta potential measurement of different formulations where lower values of zeta potential were observed for formulation MC and MH as compared to that of ML and MPL which could be again due to the high viscosity of castor oil and heavy liquid paraffin, since all other factors including the amount of cross-linking agent were kept constant, and hence, the difference is due to the change in the viscosity of different oil phases taken. Moreover, lower values of zeta potential may also have contributed to aggregation in case of MC and MH. One can clearly visualize the layer of oil covering the microspheres of formulation MC and MH (Fig. 1a, b). Since oil itself is nonconductor and also covers the surface containing the free amino groups due to its sticky and viscous nature which results in very low zeta potential. There is very less difference between zeta potential of formulation ML and MPL. This possibly could be due to the loose aggregates formed in the ML formulation, which resisted the movement of particles in electrostatic field as against the free-flowing microspheres in the case of formulation MPL.
Percent drug entrapment was lower for the microspheres prepared in castor oil and heavy liquid paraffin, and it was observed that it increased with the increase in the uniformity of the microspheres and was more in case of microspheres prepared in light paraffin and the mixture of light paraffin and petroleum ether. This could be explained in terms of the process yield where the total amount of the polymer may not have resulted into microspheres (polymer aggregates and large clumps which cannot be considered as microspheres), thus leading to less drug entrapment as is the case with the microspheres prepared in the more viscous oil phase. There is very less difference between percent drug entrapment of formulation ML and MPL. This effect may be attributed to low difference between their oil phase viscosities.
The effect of viscosity on the properties of microspheres can be visualized through in vitro drug release pattern of different formulations in a much better way. It was noticed that microspheres prepared in castor oil (formulation MC) exhibited no release at the beginning and was very slow till the last point of the study. This can be attributed to the highly viscous oil layer, which acted as impermeable barrier for drug diffusion into the dissolution media. In the case of formulation MH, the release was slow and increased gradually at very slow pace. This could be due to larger microspheres with less viscous oil layer adhered to their surface, which could have been easily eroded. There was very little difference between the drug release profile of the formulation ML and MPL as both formulations witnessed an initial faster release of the drug, which could be due to the burst effect as is generally observed with such type of the particulate or matrix systems. A little faster and more percent drug release from formulation MPL as compared to ML may be due the fact that the former existed in monodisperse form with more surface area exposed to dissolution media and hence, the effect. Moreover, aggregation (as seen clearly in case of formulations MC, MH, and partial aggregation in case of ML) prevents transportation of PBS to the microspheres, which also slows down the drug release. The statistical analysis of the parameter “percent drug release” from 0 to 7 h indicated that the viscosity of different oil phases has a significant effect on the in vitro release of the drug from different formulations prepared utilizing different oil phases (analysis of variance, P < 0.05). The reason for this difference has already been explained in this section itself.
CONCLUSIONS
The effect of viscosity of external phase on the characteristics of chitosan microspheres was observed in the present work. It was noted that viscosity of the external phase plays an important role in the development of microspheres prepared by emulsification technique. The same affects many attributes and characteristics of the formulation. Proper selection of external phase during the process of optimization would save the process time and chemicals consumed. Hence, viscosity of the oil phase must be properly regulated to develop proper and efficient delivery system to make them pharmaceutically acceptable. Probably, the work would lend support to the researchers associated with formulation and development of matrix-based microparticulate drug delivery systems.
Acknowledgments
Financial support from Indian Council of Medical Research (ICMR), Government of India-New Delhi, India for the work is gratefully acknowledged.
Authors are thankful to All India Institute of Medical Sciences-New Delhi, India for scanning electron microscopy and Director, CIFT, Cochin, India for generous gift of chitosan.
References
- 1.Woo BH, Jiang G, Jo YW, Deluca PP. Preparation and characterization of a composite PLGA and poly(acrylolyl hydroxymethyl starch) microsphere system for protein delivery. Pharm Res. 2001;18:1600–6. doi: 10.1023/A:1013090700443. [DOI] [PubMed] [Google Scholar]
- 2.Capan Y, Jiang G, Giovagnoli S, Deluca PP. Preparation and characterization of poly (D, l-lactide-co glycolide) microsphere for controlled release of human growth hormone. AAPS PharmSciTech. 2003;4:E28. doi: 10.1208/pt040228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gohel MC, Amin AF. Formulation optimization of controlled release of diclofenac sodium microspheres using factorial design. J Control Rel. 1998;51:115–22. doi: 10.1016/S0168-3659(97)00102-8. [DOI] [PubMed] [Google Scholar]
- 4.Vasir JK, Tambwekar K, Garg S. Bioadhesive microspheres as a controlled drug delivery system. Int J Pharm. 2003;255:13–32. doi: 10.1016/S0378-5173(03)00087-5. [DOI] [PubMed] [Google Scholar]
- 5.Muzzarelli RAA. Chitin. New York: Academic; 1977. [Google Scholar]
- 6.Mima S, Yoshikawa S, Mima M. Chitosan reinforced polyvinyl alcohol membranes. Jpn Pat. 1985;130:870. [Google Scholar]
- 7.Qurashi MT, Blair HS, Allen SJ. Studies on modified chitosan membranes. I. Preparation and characterization. J Appl Poly Sci. 1992;46:255–61. doi: 10.1002/app.1992.070460206. [DOI] [Google Scholar]
- 8.Kibune K. Digestible chitin sutures. Jpn Pat. 1980;128:160. [Google Scholar]
- 9.Balassa LL. Chitin containing wound healing composition. Br Pat. 1971;1252:373. [Google Scholar]
- 10.Malette WG, Quigley HJ, Gaines RD, Johnson ND, Rainer WG. Chitosan: a new haemostatic. Ann Thorac Surg. 1983;36:55–8. doi: 10.1016/S0003-4975(10)60649-2. [DOI] [PubMed] [Google Scholar]
- 11.Kaifu K, Komai T. Wetting characteristics and blood clotting on surface of acylated chitins. J Biomed Mater Res. 1982;16:757–66. doi: 10.1002/jbm.820160602. [DOI] [PubMed] [Google Scholar]
- 12.Muzzarelli R, Baldassarre V, Conti F. Biological activity of chitosan: ultrastructural study. Biomaterials. 1988;9:247–2. doi: 10.1016/0142-9612(88)90092-0. [DOI] [PubMed] [Google Scholar]
- 13.Hirano S, Noishiki Y. The blood compatibility of chitosan and N-acetyl chitosan membranes. J Biomed Mater Res. 1985;19:413–7. doi: 10.1002/jbm.820190406. [DOI] [PubMed] [Google Scholar]
- 14.Chandy T, Sharma CP. Chitosan—as a biomaterial. Biomater Artif Cells Artif Organs. 1990;18:1–24. doi: 10.3109/10731199009117286. [DOI] [PubMed] [Google Scholar]
- 15.Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as novel nasal delivery system for vaccines. Adv Drug Del Rev. 2001;51:81–96. doi: 10.1016/S0169-409X(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 16.Sawayanagi Y, Nambu N, Nagai T. Use of chitosan for the sustained release preparation of water soluble drugs. Chem Pharm Bull. 1982;30:4213–5. doi: 10.1248/cpb.30.4213. [DOI] [PubMed] [Google Scholar]
- 17.Nagai T, Sawayanagi Y, Nambu N. Application of chitin and chitosan to pharmaceutical preparations. In: Zikakis JP, editor. Chitin, chitosan and related enzymes. New York: Academic; 1984. pp. 21–39. [Google Scholar]
- 18.Kawashima Y, Handa T, Kasi A, Takeneka H, Lin SY, Ando Y. Novel method for the preparation of controlled release theophylline granules coated with a polyelectrolyte complex of sodium polyphosphate chitosan. J Pharm Sci. 1985;74:264–8. doi: 10.1002/jps.2600740308. [DOI] [PubMed] [Google Scholar]
- 19.Ouchi T, Bamba T, Fugimoto M, Hamamoto S. Synthesis and anti tumor activity of chitosan carrying 5-fluorouracils. Makromol Chem. 1989;190:1817–25. doi: 10.1002/macp.1989.021900807. [DOI] [Google Scholar]
- 20.Wantanabe K, Saiki I, Uraki Y, Tokura S, Azuma I. 6-O carboxymethylchitin as a drug carrier. Chem Pharm Bull. 1991;38:506–9. doi: 10.1248/cpb.38.506. [DOI] [PubMed] [Google Scholar]
- 21.Ohya Y, Takei T, Kobayashi H, Ouchi T. Release behaviour of 5- fluorouracil from chitosan-gel microspheres immobilizing 5-fluorouracil derivative coated with polysaccharides and their cell specific recognition. J Microencapsulation. 1993;10:1–9. doi: 10.3109/02652049309015307. [DOI] [PubMed] [Google Scholar]
- 22.Shiaraishi S, Imai T, Otagiri M. Controlled release of indomethacin by chitosan-polyelectrolyte complex: optimization and in vivo/in vitro evaluation. J Control Rel. 1993;25:217–25. doi: 10.1016/0168-3659(93)90080-O. [DOI] [Google Scholar]
- 23.Thanoo BC, Sunny MC, Jayakrishnan A. Cross linked chitosan microspheres: preparation and evaluation as a matrix for the controlled release of pharmaceuticals. J Pharm Pharmcol. 1992;44:283–6. doi: 10.1111/j.2042-7158.1992.tb03607.x. [DOI] [PubMed] [Google Scholar]
- 24.Majithiya RJ, Murthy RSR. Chitosan based mucoadhesive microspheres of clarithromycin as a delivery system for antibiotic to stomach. Curr Drug Del. 2005;2:235–42. doi: 10.2174/1567201054367995. [DOI] [PubMed] [Google Scholar]
- 25.Patel JK, Patel RK, Amin AF, Patel MM. Formulation and evaluation of mucoadhesive glipizide microspheres. AAPS PharmSciTech. 2005;6(1):E49–55. doi: 10.1208/pt060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehr CM, Bowstra JA, Tukker JJ, Junginger HE. Intestinal transit of bioadhesive microspheres in an in situ loop in the rat. J Control Rel. 1990;13:51–62. doi: 10.1016/0168-3659(90)90074-4. [DOI] [Google Scholar]
- 27.Rhodes CT. Disperse systems. In: Banker GS, Rhodes CT, editors. Modern pharmaceutics. 2. New York: Marcel Dekker; 1990. p. 333. [Google Scholar]
- 28.Wang LY, Ma GH, Su ZG. Preparation of uniform sized microspheres by membrane emulsification technique and application as a carrier of protein drug. J Control Rel. 2005;106:62–75. doi: 10.1016/j.jconrel.2005.04.005. [DOI] [PubMed] [Google Scholar]