INTRODUCTION
In vitro dissolution testing is important for providing process control and quality assurance, determination of stability release characteristics of the product over time, and facilitating certain regulatory determinations (e.g., absence of effect of minor formulation changes or of change in manufacturing site on performance). In the past two decades, the dissolution test has also been widely discussed to serve as an indicator of how the formulation will perform in vivo (1–3).
Although basket and paddle methods are currently the most popular methods for many drug products, both methods are operated under closed finite sink condition and cannot mimic the conditions present in the digestive system. A flow-through method using the official USP 4 apparatus operated in open-loop mode is capable of maintaining a continuous flow of fresh dissolution medium, thus, maintaining infinite sink conditions (3–6). This operational mode provides an environment potentially closer to that of the digestive tract. Thus, this technique may offer advantages for establishing in vitro and in vivo correlations (IVIVCs).
To compare dissolution testing under finite and infinite sink conditions, this study applied both paddle and flow-through methods for dissolution testing with disintegrating prednisone and nondisintegrating salicylic acid tablets. The closed- and open-loop configurations of the flow-through method were used to provide comparisons with the paddle method.
In addition to studies based on the official USP dissolution apparatus, several authors have reported development of novel, multicompartment dissolution apparatus in their efforts to develop good IVIVCs (7–9; Hughes et al., private communication). The reported novel systems have potential to simulate in vivo condition such as gastric volume and pH, gastric emptying to the intestine, and intestinal volume and pH etc., which may give a good in vivo and in vitro correlation. However, these novel apparatus require special configurations or parts, which may be difficult to duplicate in other labs. This study applies the idea of a multicompartment dissolution apparatus but attempts to minimize modifications to the current official USP apparatus so as to facilitate adoption by other labs for application to their specific studies. In this study, the flow-through apparatus with open-loop configuration has been modified by adding a flow-through reservoir after the flow-through cell. This reservoir is maintained at intestinal pH. Disintegrating prednisone and nondisintegrating salicylic acid were used as test drugs.
EXPERIMENTAL
Materials
NCDA#2, 10 mg prednisone tablets were used as an example of a disintegrating tablet (10). Salicylic acid tablets (300 mg; USP, Lot M) were used as an example of a nondisintegrating tablet.
Dissolution Methods
Paddle Methods
A USP apparatus 2 (Distek dissolution apparatus Model 2100A, Serial number D12547192, North Brunswick, New Jersey 08902, mechanically calibrated according to Gao et al. 11) was employed. Prednisone tablet dissolution was carried out in 500 ml water, and 900 ml water was used when testing the salicylic acid tablet. All dissolution runs were carried out at 37°C and an agitation speed of 50 rpm.
Flow-Through Open-Loop and Closed-Loop Methods
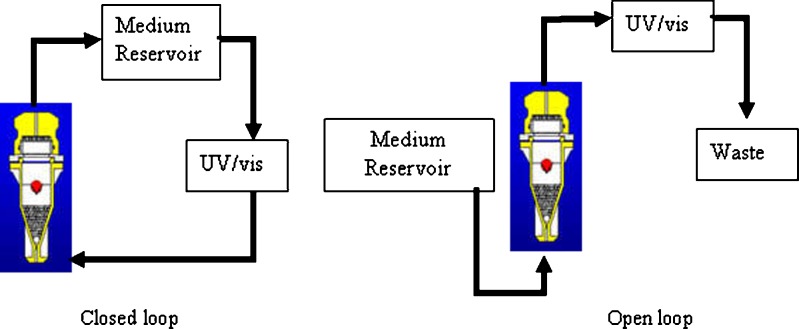
A USP apparatus 4 (CE 7 Smart with CP7 piston pump, Sotax AG, Switzerland) with 22.6 mm (Sotax part number 8820) flow cells was used during the study. Each cell was prepared by placing a 5-mm ruby bead in the apex of the cone to protect the inlet tube, and 8.0 g of 1 mm glass beads were added to the cone area to form a glass bead bed. The tablet sample was positioned on the glass bead bed. Mechanical calibration consists of measuring the flow rate through each cell. The temperature of the flow cell unit was 37.0 ± 0.5°C. The closed- and open-loop configurations are shown in Fig. 1. When testing with the closed loop, 500 ml water was used for the prednisone tablet and 900 ml water for salicylic acid tablet.
Fig. 1.
USP 4 method with closed- and open-loop configuration
Modified Flow-Through Methods (Open Loop)
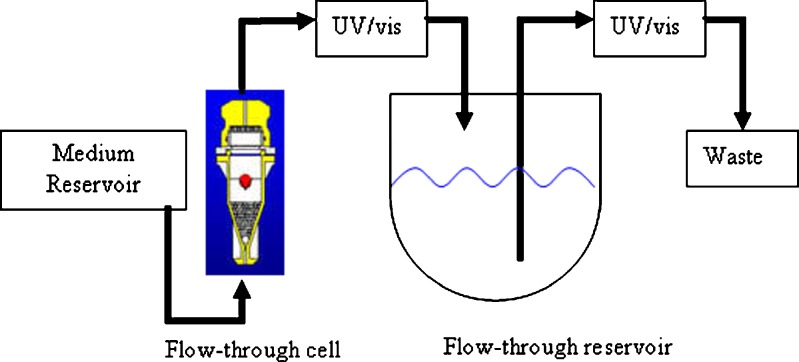
The modified USP apparatus 4 is shown in Fig. 2. The flow-through cell (the standard USP apparatus 4 flow cell) is set up according to the official USP method for the drug being analyzed and the drug sample is inserted (12). In the modified apparatus, instead of flowing directly into a waste container, the effluent dissolution medium from the flow-through cell flows into a reservoir, which contains a predetermined volume of simulated intestinal fluid. The rates of flow in and out of the reservoir are equal, and thus, its volume remains constant throughout the dissolution test. In this study, the flow-through reservoir contains 500 ml water when testing prednisone tablets and 900 ml water when testing salicylic acid tablets. The medium temperature was kept at 37.0 ± 0.5°C. Deionized water used in the studies was degassed as described by Gao et al. (11).
Fig. 2.
Diagrammatic representation of apparatus 4 as modified for this study
Sample Analysis
Sample analysis was performed by following the solution absorbance (Agilent 8453 spectrophotometer, Santa Clara, California 95051, United States). Prednisone samples were analyzed at 242 nm with a 0.5 cm cell, and salicylic acid samples were analyzed at 296 nm with a 0.2 cm cell.
For experiments using a paddle method, all samples for analysis were filtered through Distek 10 µm ultra high molecular weight polyethylene filter tips attached to the top of the Distek sampling probes (filters are not in the dissolution medium). The metal sampling probes were left in the medium throughout the run. Reported dissolution values represent the average value for six vessels from one run.
With the flow-through method, all samples for analysis were filtered through glass microfiber filters (Whatman, GF/D, Cat# 1823-025). Reported dissolution values represent the average value for at least three cells from one run.
With the modified flow-through method, samples from the flow-through cell were filtered through glass microfiber filters (Whatman, GF/D, Cat# 1823-025), and samples from the flow-through reservoir were filtered through Distek 10 µm ultra high molecular weight polyethylene filter tips. Reported dissolution values represent the average value for at least three cells from one run.
RESULTS AND DISCUSSION
Comparison of Closed-Loop Flow-Through Methods with Paddle Methods
Both the closed-loop configuration for the flow-through and the paddle method operate under finite sink conditions. Differences observed between results produced by these two methods are largely due to differences in the hydrodynamic conditions which exist around the sample. The paddle apparatus generates shear forces around samples via agitation of the bulk medium, while for the flow-through apparatus, shear is provided by the pulsatile flow over the particles (5).
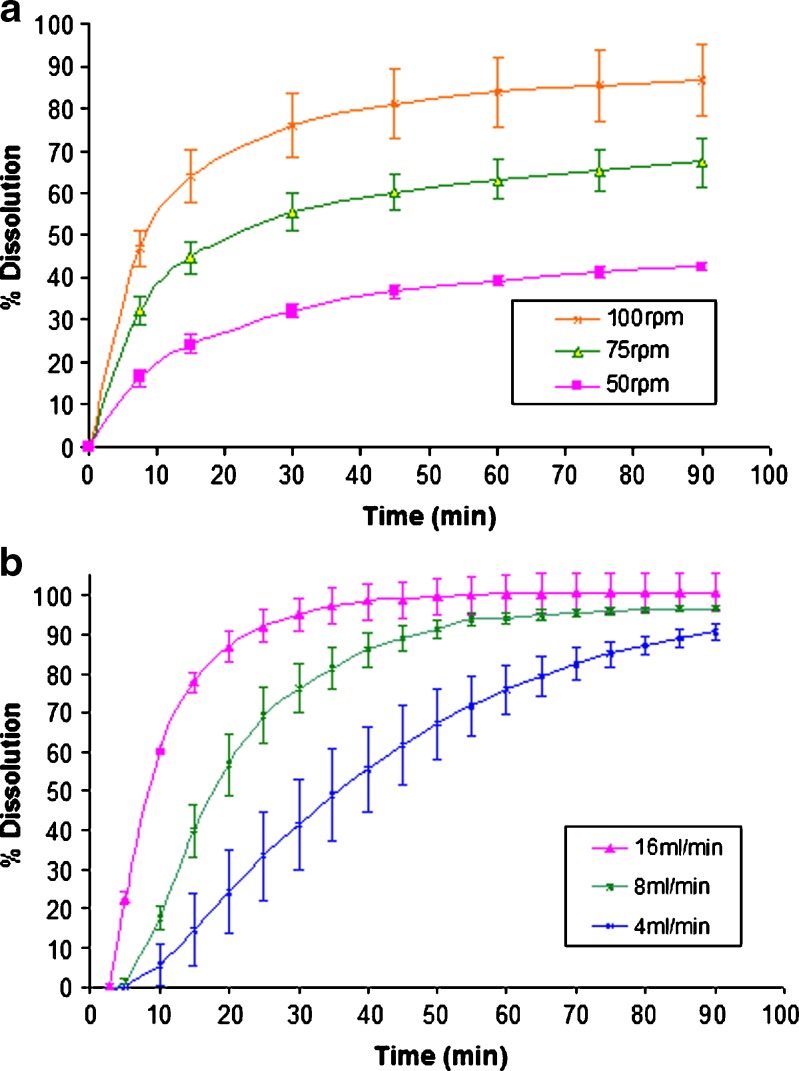
Disintegrating (10 mg prednisone) and nondisintegrating (300 mg salicylic acid) types of tablets were used as model drugs and tested using both paddle and closed-loop flow-through methods. Various agitation speeds and flow rates were applied during the tests. The results are shown in Figs. 3 and 4.
Fig. 3.
Effect of paddle rotation speed and flow rate on dissolution profiles for 10 mg prednisone tablets. a paddle and b closed-loop flow-through method
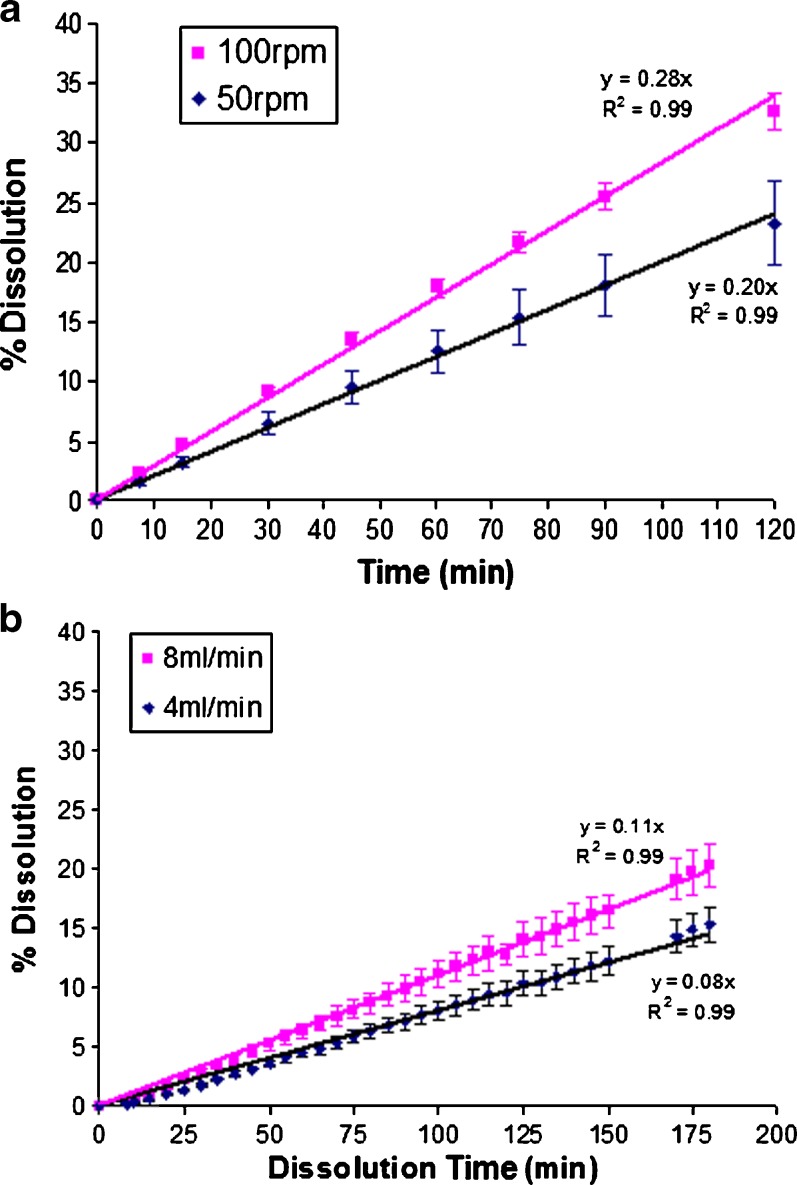
Fig. 4.
Effect of paddle rotation speed and flow rate on dissolution profiles for 300 mg salicylic acid tablets. a paddle method and b closed-loop flow-through method
With disintegrating prednisone tablets, results in Fig. 3 indicate that rate and extent of dissolution increases with increasing agitation speed (paddle apparatus) or flow rate (flow-through apparatus), but the shape of the profile is different for the two apparatus. For the paddle apparatus, when the tablet is dropped into the vessel containing medium, disintegration occurs in less than 5 min and gradually, a cone of drug substance forms in the center area if the agitation speed is below 100 rpm. The drug is trapped in this cone and is released slowly via diffusion. It follows then that if the agitation rate increases, the shear force around the cone increases, resulting in an increase of the dissolution rate. On the other hand, when prednisone disintegrates in a flow-through cell, instead of forming a cone, a layer of particles is observed on the glass bead bed. Because the dissolution medium can flow through this thin particle layer, faster drug dissolution occurs. These results show the disintegrated particles respond to the different hydrodynamic conditions in the USP paddle and USP flow-through apparatuses.
With nondisintegrating salicylic acid tablets, there are no effects due to tablet disintegration and the paddle method (Fig. 4a) and closed-loop flow-through method (Fig. 4b); both produced linear dissolution profiles (R2 > 0.99). With the paddle method using 50 and 100 rpm of paddle speed, the dissolution rates were 0.20% and 0.28%/min, respectively, and with the flow-through method, the dissolution rates were 0.08% and 0.11%/min at flow rates of 4 and 8 ml/min. Since salicylic acid tablets remain intact throughout the dissolution test, the shear forces are applied only to the surface of the tablet. Thus, dissolution in both the paddle method and the closed-loop flow-through method is zero order. The dissolution rates show that medium flowing through the sample, even at 8 ml/min (0.11%/min), provides relative mild shear force comparing with paddle agitation speed at 50 rpm (0.2%/min).
Application of Flow-Through Method with Open-Loop Configuration
The open-loop configuration is especially useful for low-solubility drugs as it can easily provide an infinite sink condition by maintaining a continuous flow of fresh dissolution medium (Fig. 1). Dissolution results obtained from the open system are typically expressed as the instantaneous drug concentration, while results from closed systems are usually expressed as the cumulative amount of drug dissolved in the medium (or a percentage of the total amount in the tablet).
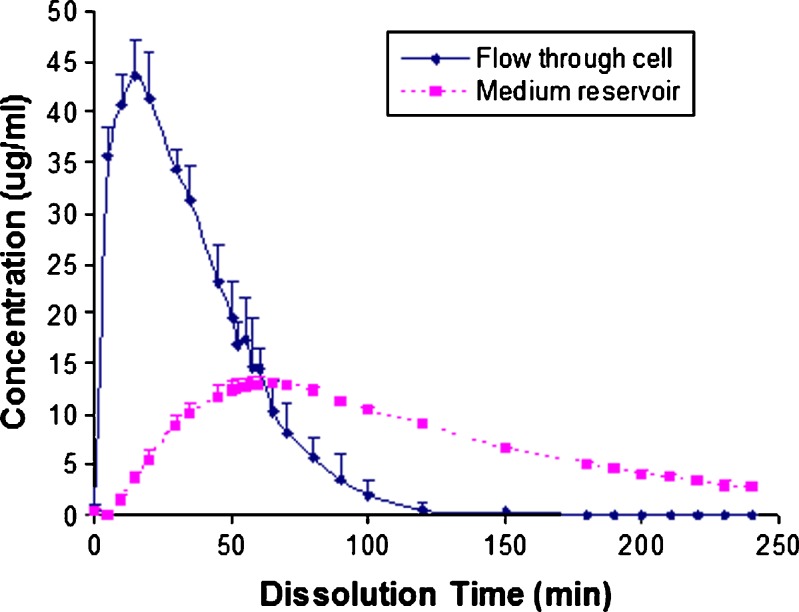
Dissolution results of prednisone and salicylic acid tablets using the flow-through apparatus with open-loop configuration at 4 ml/min flow rate are shown by the solid lines in Figs. 5 and 6, respectively. For the disintegrating prednisone tablet, a peak concentration was reached at about 20 min, and the tablet had completely dissolved in 2 h. This total dissolution time result is in agreement with that obtained for the closed-loop method (cumulative percentage dissolution results) where more than 90% prednisone dissolved after 90 min (shown in Fig. 3b at 4 ml/min flow rate).
Fig. 5.
Dissolution profiles for prednisone tablets using flow-through method with an open-loop configuration and a modified flow-through method. Flow rates were kept at 4 ml/min for both methods
Fig. 6.
Dissolution profiles for salicylic acid tablets using a flow-through method with an open-loop configuration and a flow-through method. Flow rates were kept at 4 ml/min for both methods
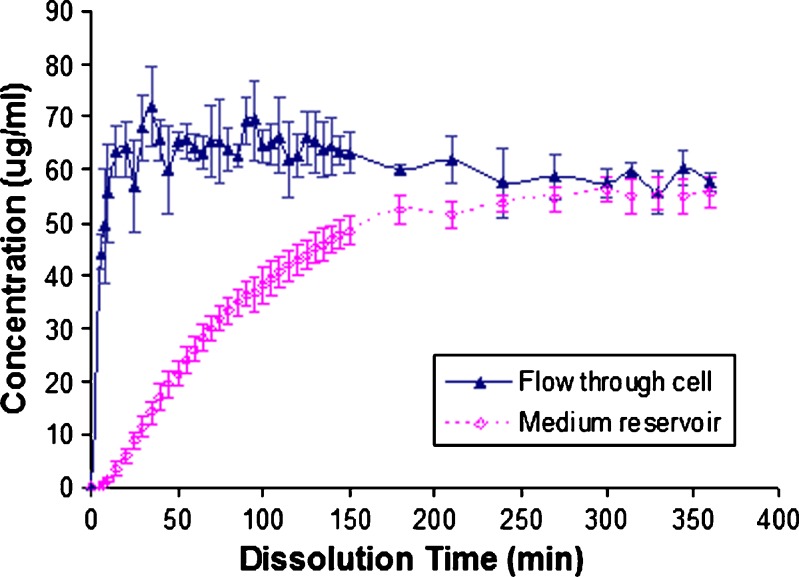
For nondisintegrating drug form, the concentration (solid line in Fig. 6) of salicylic acid tablets reaches a plateau at around 60 μg/ml shortly after starting the test. To aid comparison of open-loop results with closed-loop results, the dissolution rates (expressed as %/min) have been calculated based on the results obtained from the closed- and open-loop methods. With the closed-loop method at flow rate of 4 ml/min, the dissolution rate can be expressed by the slope in Fig. 4b (0.08%/min). The dissolution rate for the open-loop method was estimated using the following equation.
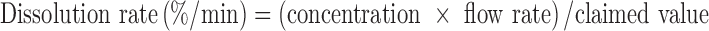 |
We observe a final concentration of 60 μg/ml for the open-loop configuration. The label claim value for the salicylic acid tablets is 300 mg. The calculated dissolution rate for salicylic acid at 4 ml/min for this configuration is about 0.08%/min, the same value to that of the closed-loop configuration for this highly soluble nondisintegrating drug.
Application of Modified Open-Loop Flow-Through Method
Various attempts have been made to establish correlations between in vitro dissolution results and in vivo plasma concentrations. Most of these efforts involve fitting data acquired using an official USP dissolution apparatus to various mathematical models (1). Because available technology may not always permit measurements leading to a meaningful IVIVC, there is a motivation to design dissolution apparatus to better simulate the digestive system, typically by including multiple compartments (gastric, intestinal, and absorption or clearance). Although results from reported new systems are promising, all are based on new technologies that are not commercially available.
The current USP apparatus 4 with its flow-through mechanism has potential for GI tract simulations. In this study, by applying the same concepts as used in the reports mentioned above and adding a flow-through reservoir to the open-loop configuration, the apparatus depicted in Fig. 2 was constructed. The advantages of this design are (1) adoption of flow-through method with open-loop configuration to more easily simulate the GI tract and (2) easier application in other laboratories since it is based on commercially available apparatus.
Two model drugs, prednisone and salicylic acid, were used to test this modified flow-through apparatus. The dotted curves in Figs. 5 and 6 show concentrations of prednisone and salicylic acid in the flow-through reservoir.
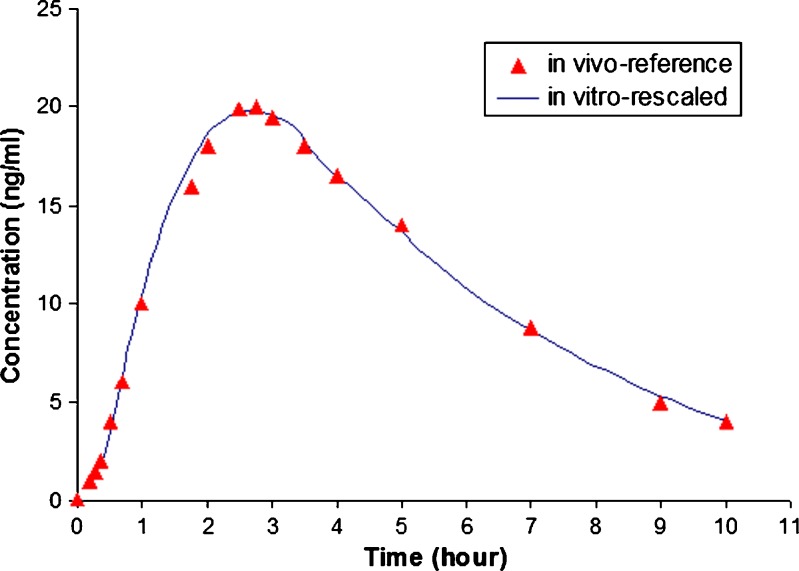
Prednisone is a BCS I drug (13) formulated as a fast disintegrating tablet. Its dissolution was rapid in the flow-through cell with a maximum concentration obtained in about 20 min as shown in Fig. 5 (solid line). The effluent dissolution medium from the flow-through cell was pumped into the reservoir to simulate the GI tract. Since BCS I drugs exhibit high permeation, the drug concentration in flow-through reservoir may also correlate with the plasma concentration. The in vitro pharmacokinetic parameters including maximum concentration (Cmax), time to reach the maximum concentration (Tmax) and area under the concentration–time curve (AUC) for prednisone obtained using this apparatus are listed in Table I. For comparison, the reference pharmacokinetic results from in vivo studies are also listed in Table I (14,15). The Tmax value obtained using the modified flow-through apparatus is in the range of those reported for the in vivo studies (13), but the Cmax and AUC are quite different. Considering the differences in volume between that used for dissolution testing and the nominal volume of the GI tract or blood, as well as the flow rate and drug transit time in the GI tract, it is necessary to incorporate factors to adjust for these differences. The dissolution profile obtained from the flow-through reservoir in this study was rescaled by applying two factors to adjust time (Ft) and concentration (Fc). The rescaling factors were obtained from the Tmax and Cmax ratios for in vivo and in vitro data. For prednisone in this study, the Ft is 2.5 and Fc is 0.0015. Applying these two factors to the in vitro data result in the rescaled profile shown in Fig. 7. These rescaled in vitro data have good correlation with the in vivo profile.
Table I.
Comparison of In Vitro Pharmacokinetic Data for Prednisone Obtained Using the Modified USP Apparatus 4 with Data Obtained from Various References
| C max (ng/ml) | T max (hour) | AUC | ||
|---|---|---|---|---|
| Modified USP 4 results (10 mg prednisone tablet) | Experimental | 13,000 | 1.1 | 29,170 |
| Rescaled | 19.5 | 2.8 | 109 | |
| In vivo results based on reference (13) | 80–100% availability | 1–3 | − | |
| In vivo results based on reference (15) (20 mg prednisone tablet) | Manufacturer A | 42 | 2.5 | 268 |
| Manufacturer B | 42 | 2.4 | 266 | |
| Manufacturer C | 39 | 2.2 | 254 | |
| Manufacturer D | 39 | 2.2 | 264 | |
| Manufacturer E | 38 | 2.2 | 254 | |
Fig. 7.
Comparison of rescaled dissolution profile obtained from modified flow-through reservoir with in vivo data from reference (15)
Because pharmacokinetic parameters for the studied 300 mg salicylic acid tablet could not be found, it is not possible to compare our results with in vivo data.
The results presented here indicate that it may be possible to obtain meaningful pharmacokinetic parameters using the described modification of the open-loop configuration flow-through apparatus. This modified USP dissolution apparatus may provide a feasible way to achieve simple model IVIVCs by adjusting the medium volume in the flow-through reservoir, medium rates, and medium pH or composition.
CONCLUSIONS
The flow-through method with closed- and open-loop configurations has been studied and compared with the paddle method. The results based on disintegrating and nondisintegrating tablets show that the flow-through apparatus with closed-loop configuration provides similar dissolution profiles as those obtained using the paddle method. The dissolution rates obtained using the two configurations are similar if the sink condition is maintained.
The modified USP flow-through apparatus with open-loop configuration shows potential for correlation with in vivo data and can be easily set up and applied by other labs. Preliminary results indicate that the modified method may be able to provide simple model IVIVCs for the prednisone tablet. Studies are currently ongoing to validate the modified flow-through apparatus as well as extend these studies to additional drug products.
References
- 1.Guidance for Industry, Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations, FDA/CDER, September 1997. [DOI] [PubMed]
- 2.Dressman J, Kramer J. Pharmaceutical dissolution testing. Boca Raton: Taylor & Francis; 2005. [Google Scholar]
- 3.Cohen JL, Hubert BB, Leeson LJ, Rhodes CT, Robinson JR, Roseman TJ, Shefter E. The development of USP dissolution and drug release standards. Pharm Res. 1990;7(10):983–7. doi: 10.1023/A:1015922629207. [DOI] [PubMed] [Google Scholar]
- 4.Nicklasson M, Wennergren B, Lindberg J, Persson C, Ahlgren R, Palm B, Pettersson A, Wenngren L. A collaborative in vitro dissolution study using the flow-through method. Int J Pharm. 1987;37:195–202. doi: 10.1016/0378-5173(87)90029-9. [DOI] [Google Scholar]
- 5.Wennergren L, Lindberg L, Nicklasson M, Nilsson G, Nyberg G, Ahlgren R, Persson C, Palm B. A collaborative in vitro dissolution study: comparing the flow-through method with the USP paddle method using USP prednisone calibrator tablets. Int J Pharm. 1989;53:35–41. doi: 10.1016/0378-5173(89)90358-X. [DOI] [Google Scholar]
- 6.Nicklasson M, Orbe A, Lindberg J, Borgh B, Magnusson A, Nilsson G, Ahlgren R, Jacobsen L. A collaborative study of the in vitro dissolution of phenacetin crystals comparing the flow through method with the USP Paddle method. Int J Pharm. 1991;53:35–41. [Google Scholar]
- 7.Kobayashi M, Sada N, Sugawara M, Iseki K, Miyazaki K. Development of a new system for prediction of drug absorption that takes into account drug dissolution and pH change in the gastro-intestinal tract. Int J Pharm. 2001;221:87–94. doi: 10.1016/S0378-5173(01)00663-9. [DOI] [PubMed] [Google Scholar]
- 8.Gu C, Rao D, Gandhi R, Hilden J, Raghavan K. Using a novel multicompartment dissolution system to predict the effect of gastric pH on the oral absorption of weak bases with poor intrinsic solubility. J Pharm Sci. 2005;94:199–208. doi: 10.1002/jps.20242. [DOI] [PubMed] [Google Scholar]
- 9.Parikh R, Parikh D, Delvadia R, Patel S. A novel multicompartment dissolution apparatus for evaluation of floating dosage form containing poorly soluble weakly basic drug. Disso Tech. 2006;2:14–9. [Google Scholar]
- 10.Moore TW, Shangraw RF, Habib Y. Dissolution calibrator tablets: a recommendation for new calibrator tablets to replace both current USP calibrator tablets. Pharm Forum. 1996;22(3):2423–8. [Google Scholar]
- 11.Gao Z, Moore TW, Doub WH, Westenberger BJ, Buhse LF. Effects of deaeration methods on dissolution testing in aqueous media: a study using a total dissolved gas pressure meter. J Pharm Sci. 2006;92(7):1606–13. doi: 10.1002/jps.20622. [DOI] [PubMed] [Google Scholar]
- 12.The United States Pharmacopeia, USP 27: Physical Tests, < 724 > Drug Release, “Apparatus 4 (Flow-Through Cell)”, p2307, 2004.
- 13.Vogt M, Derendorf H, Krämer J, Junginger H, Midha K, Shah V, Stavchansky S, Dressman J, Barends D. Biowaiver monographs for immediate release solid oral dosage forms: Prednisolone. J Pharm Sci. 2007;96:1–10. doi: 10.1002/jps.20727. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan T, Sakmar E, Albert K, Blair D, Wagner J. In Vitro and in vivo availability of commercial prednisone tablets. J Pharm Sci. 1975;64:1723–5. doi: 10.1002/jps.2600641035. [DOI] [PubMed] [Google Scholar]
- 15.Francisco C, Honigberg I, Stewart J, Kotzan J, Brown W, Schary W, Pelsor F, Shah V. In vitro and in vivo bioequivalence of commercial prednisone tablets. Biopharm Drug Dispos. 1984;5:335–44. doi: 10.1002/bdd.2510050405. [DOI] [PubMed] [Google Scholar]