Fig. 1.
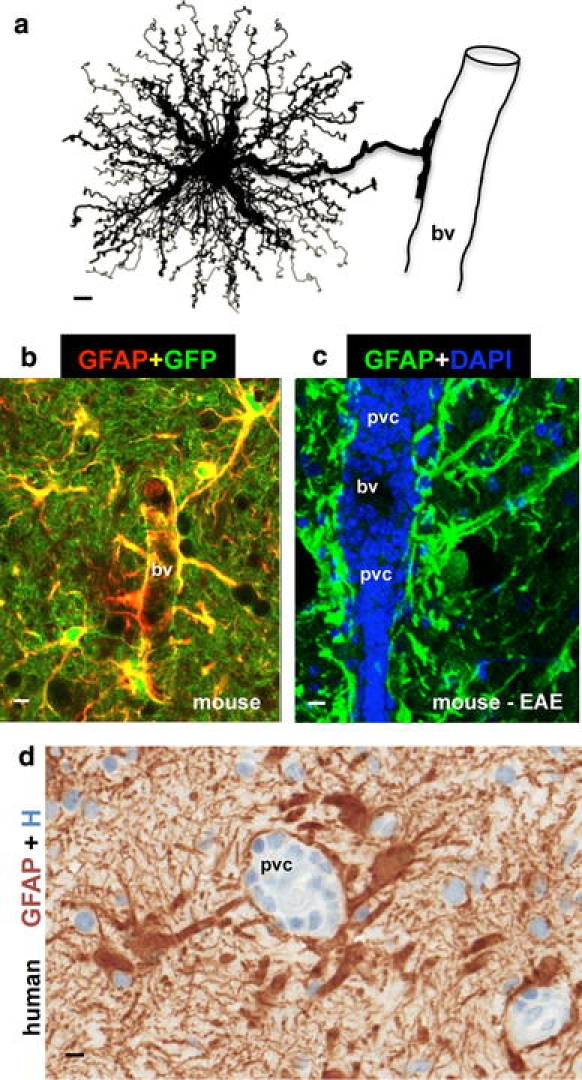
Astrocyte morphology and interactions with blood vessels in healthy and diseased tissue. a Protoplasmic astrocyte giving rise to a dense network of finely branching processes throughout its local gray matter neuropil, as well as to a large stem branch that extends foot processes along a blood vessel (bv). b Two color fluorescence showing astrocytes in healthy mouse gray matter stained immunohistochemically for GFAP (red) as well as the transgene-derived reporter molecule GFP (green). Note that in these transgenic mice [95], GFP reporter is present in all of the fine processes of the protoplasmic astrocytes throughout the neuropil, whereas the GFAP is present only in the large stem astrocyte processes and endfeet (which appear yellow where green and red staining overlap). Note that endfeet from many astrocytes contact and envelop bv. c Two color fluorescence showing dense accumulations of GFAP-positive (green) endfeet and processes of reactive astrocytes lining up along perivascular cuffs or clusters (pvc) of inflammatory cells stained with DAPI (blue) in a mouse with experimental autoimmune encephalomyelitis (EAE). Transgenic disruption of this reactive astrocyte barrier leads to widespread invasion of inflammatory cells away from perivascular clusters into CNS parenchyma during EAE [251]. d Two color brightfield staining showing human autopsy specimen with reactive astrocytes lining their processes along perivascular cuffs of inflammatory cells as if forming perivascular scar-like barriers similar to those observed in experimental animal models [251]. Scale bars a 3 μm, b 7.5 μm, c 15 μm, d 5 μm
