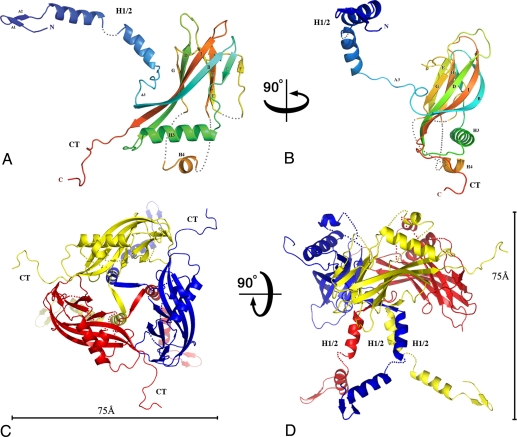
Fig. 2.
Structure of the baculovirus polyhedrin protein. Cartoon representations of the WNPV polyhedrin molecule (A and B) and its trimeric assembly (C and D) in orthogonal views. In A and B, the polypeptide chain is colored in a blue-to-red gradient from the N-terminus to the C-terminus (CT). The polyhedrin has a jelly-roll fold with two sheets, BIDG and FCHE. Helix H1/2 and the CT loop extend away from the main body of the trimer in orthogonal directions. Three regions are disordered, and dotted lines represent the missing loops. First, only weak electron density was visible for the H1/2 helix so that only the main chain was traced with a short break between residues 37 and 39. Second, the short loop comprising residues 142–145 had no interpretable electron density and was not modeled. Third, the region comprising residues 171–202 was disordered apart from a short helix (186–194). (C and D) Trimer is represented in orthogonal views, with scale bars representing 75 Å. The trimer has the shape of a chalice with a tripod base (H1/2) and prominent protrusions (CT).