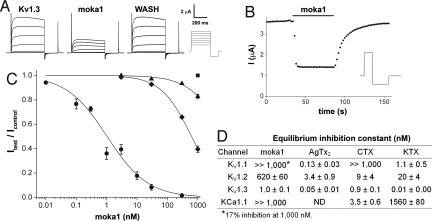
Fig. 3.
Moka1, a high-affinity and specific Kv1.3 channel blocker. Ion channel isoforms expressed in Xenopus laevis oocytes and studied by two electrode voltage clamp included rat Kv1.1, rat Kv1.2, human Kv1.3, and mouse KCa1.1. Bath solution was (in mM): 2 KCl, 96 NaCl, 1 MgCl2, 1.8 CaCl2, 5 Hepes (pH 7.5), and 0.1% bovine serum albumin (BSA). Equilibrium inhibition was determined by fitting dose-response curves for moka1 and calculated for other toxins from percent block. kon and koff were calculated. Methods are described in depth in SI Materials and Methods. (A) Representative current traces for human Kv1.3 channels before, in the presence of, and after washout of 3 nM moka1 at steady state. (Scale bars: 2 μA and 200 ms.) Voltage protocol: holding voltage −100 mV, 500 ms steps of 15 mV from −60 mV to 30 mV followed by a 200 ms step to −135 mV with a 30-s interpulse interval. (B) The time course for block and unblock of human Kv1.3 on acute application (bar) and washout of 3 nM moka1 during 100 ms steps to 0 mV from −100 mV followed by a 200-ms step to −135 mV every 2 s. (Inset) Voltage protocol. (C) Dose-response relationships for moka1 inhibition of human Kv1.3 (●), rat Kv1.1 (▴), rat Kv1.2 (◆), and mouse KCa1.1 (■), n = 3–11 cells. Kv1.1, Kv1.2, or Kv1.3 peak currents were recorded during a 500-ms step every 30 s to 0 mV from a holding voltage of −100 mV, followed by a 200-ms step to −135 mV. KCa1.1 currents were recorded with 50-ms steps every 3 s to 60 mV from −80 mV, followed by a 40-ms step to −100 mV. (D) Measured equilibrium inhibition (nM) for moka1, KTX, AgTx2, CTX on Kv1.3, Kv1.1, Kv1.2, and KCa1.1. Ce3 is reported to block human Kv1.3 with Ki of 366 nM (5). AgTx2 is reported to block KCa1.1 with Ki >1,000 nM (Table S8). Block of human Kv1.3 in human embryonic kidney (HEK293) cells showed inhibition with an affinity at equilibrium (Ki) of 4.4 ± 0.5 nM (Table S9).