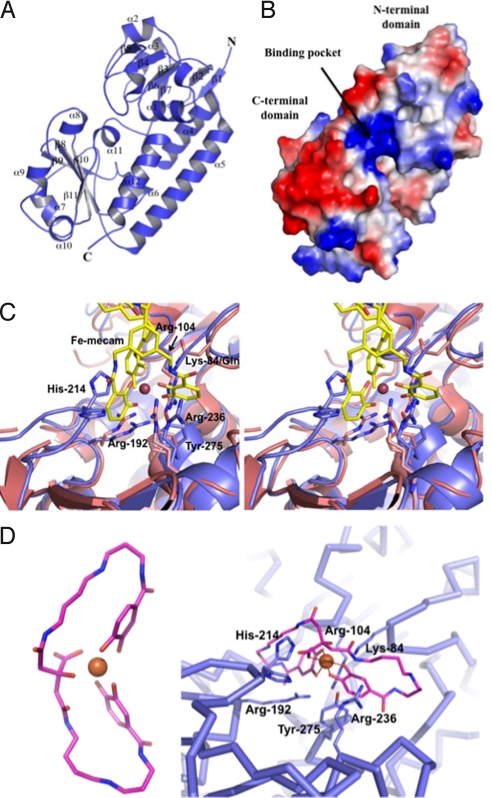
Fig. 4.
Structural analysis of YclQ. (A) Ribbon drawing of a YclQ monomer; numbering of secondary structures is shown as α1–12 and β1–11. (B) Surface charge potential of YclQ; blue indicates positive and red is for negative. Distinctively, the binding pocket is positively charged to attract [Fe(PB)]3−. (C) Binding site of YclQ shown with the superposed CeuE complexed with [Fe(mecam)2]6− in a stereoplot. The conserved residues including Arg and Tyr are shown in stick drawing with gray blue for the YclQ side chains and in magenta for the CeuE side chains; His-214 of YclQ is also shown. (D) (Left) The stick model of FePB (carbon atoms are shown in pink, oxygen atoms in orange, and nitrogen atoms in blue; Fe ion is indicated as an orange ball). (Right) FePB docked into the binding pocket of YclQ shown in an α-carbon tracing. Conserved protein residues potentially interacting with FePB are shown in dark blue sticks with labels. Near the bottom of the pocket, Arg-192 and Arg-104, with minor adjustments, can interact with hydroxyl groups of two 3,4-DHB moieties. Tyr-275 at the bottom of the pocket could stack with one of catechol rings or orient aforementioned Arg residues by contacting with OH. The carboxyl in the citrate moiety may be stabilized by a hydrogen bond with His-214. Lys-84 and Arg-236 are in the neighborhood of spermidine carbonyls to make direct contacts with FePB.