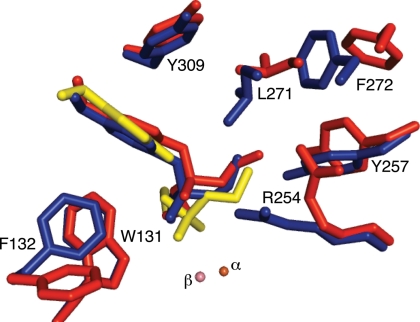
Fig. 1.
Active sites of arPTE EopenS, EclosedS, and EclosedP complexes. The structure of the EopenS complex (red) in which the phosphoryl oxygen of the substrate is 4.0 Å from the β-metal ion is from the arPTE 8M variant (PDB ID code 3A3W), which is predominantly in the Eopen conformation. The EclosedS complex (blue) in which the phosphoryl oxygen of the substrate is 3.3 Å from the β-metal ion, and the structure of EclosedP (yellow), in which the phosphoryl oxygen of the product is 2.4 Å from the β-metal ion, were superimposed in wild-type arPTE (PDB ID code 2R1N). Both Eopen and Eclosed conformations are present, in the absence of substrate, in the arPTE 4M variant. Closer contacts between enzyme and substrate the immediate active site (F132, R254, Y257, L271) in EclosedS are evident.