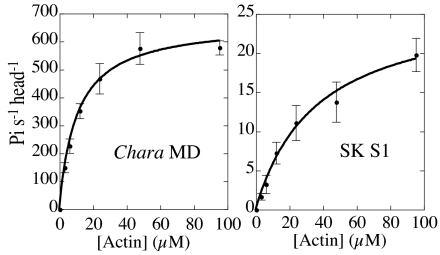
Fig. 1.
Actin-activated ATPase activities of Chara myosin motor domain and skeletal myosin subfragment-1. Values are averages of 3 measurements. Data were fitted to the Michaelis-Menten equation. Vmax of Chara myosin motor domain and skeletal myosin subfragment-1 (S1) were 670 ± 20 and 27 ± 2 Pi/s/head, respectively. Kapp values of Chara myosin motor domain and skeletal myosin S1 were 11 ± 1 and 27 ± 2 μM, respectively. Note that the ATPase assays shown in this figure were done at low salt (10 mM KCl, 4 mM MgCl2, 20 mM Hepes-KOH, pH 7.4) because the ATPase activity of skeletal myosin S1 in standard ATPase assay solution increased linearly with the increase in actin concentration and both Vmax and Kapp values could not be determined.