Abstract
The increased survival of patients with breast cancer has given rise to other problems associated with the complications of chemotherapy. One major complication is premature ovarian failure, an especially harmful outcome for women of reproductive age. This study was performed to evaluate the efficacy of GnRH agonist (GnRHa) treatment on protecting ovarian function in young breast cancer patients (30.59±5.1 yr) receiving chemotherapy after surgery. Twenty-two women were enrolled and given subcutaneous injections of leuprolide acetate (3.75 mg) every 4 weeks during chemotherapy. Follow-up laboratory tests (luteinizing hormone [LH], follicle stimulating hormone [FSH], and estradiol) were performed 1, 3, and 6 months after chemotherapy. Menstruation patterns and clinical symptoms were followed up for a mean duration of 35.6±1.7 months. FSH and LH levels were normal in all patients 6 months after completing chemotherapy (8.0±5.3, 4.4±2.7 mIU/mL, respectively). During follow-up, none of the patients complained of menopausal symptoms and 81.8% experienced recovery of menstruation. This report is the first trial of GnRHa as a treatment modality to protect ovarian function during adjuvant chemotherapy in young Korean breast cancer patients.
Keywords: Ovarian function, Drug Therapy, GnRH agonist, Breast Neoplasms
INTRODUCTION
Breast cancer, a common malignancy in women, is increasing in incidence world-wide. In Korea, 57% of breast cancer patients are younger than 50 yr of age (National Center for Health Statistics, Korea, 2001-2003), while in the United States, only 22% of patients are younger than 50 (SEER, 1993-1997). The survival rates of breast cancer patients have improved owing to earlier detection and more effective treatments (1), resulting in increased concern about the quality of post-chemotherapy life. Among a variety of long-term sequelae of chemotherapy, permanent ovarian damage is particularly important for young breast cancer survivors; this can result from hypoestrogenic symptoms as well as loss of fertility. In an effort to address these problems, investigators have attempted ovarian suppression using a gonadotropin releasing hormone agonist (GnRHa) during adjuvant chemotherapy. GnRHa has been used to interrupt oocyte maturation during meiosis to protect the ovary (2). Several trials have reported higher rates of resumption of spontaneous ovulatory cycles in patients with hematological malignancies who received both chemotherapy and GnRHa simultaneously than those who received chemotherapy alone (3, 4). The majority of these trials enrolled patients with leukemia or Hodgkin's disease, thus data regarding patients with breast cancer are still lacking, especially patients of reproductive age (5, 6). Studies involving breast cancer patients have typically broadly enrolled premenopausal patients instead of exclusively reproductive-aged women (<35 yr of age), in addition to using menstrual patterns as a marker of ovarian function. Currently, large randomized controlled trials are underway to evaluate the efficacy of ovarian suppression in women undergoing breast cancer treatment, and clinicians worldwide are anticipating the results of these trials. However, in Korea, where there is a different incidence pattern of breast cancer compared to Western countries, there have been no studies regarding the protective effects of GnRHa on ovarian function in patients of reproductive age with breast cancer. Currently, GnRHa has been evaluated as a new treatment modality for breast cancer; one study reported that chemotherapy with GnRHa improved clinical outcomes. We therefore designed this study to evaluate ovarian function after GnRHa administration to young Korean breast cancer patients receiving cytotoxic chemotherapy. Our study is unique in that 1) the upper age of patients enrolled was limited to the early 30s (mean age: 30.59±0.13 yr); 2) ovarian function was serially assessed by serum hormone levels as well as menstrual patterns; 3) this is the first trial on this subject in Korea, where the incidence of breast cancer is higher in the younger reproductive-age group than in Western countries.
MATERIALS AND METHODS
We initially recruited 34 women of reproductive age with adenocarcinoma of the breast. We excluded 10 women whose serum was not sampled on follow-up, one woman who received fewer than four injections during chemotherapy, and one woman who refused chemotherapy. The remaining 22 women completed the study protocol, first receiving primary surgical therapy, then chemotherapy, and finally radiation therapy (RT). The field of RT did not include the pelvis. All patients were younger than 35 yr and had a history of normal ovarian function (follicle stimulating hormone [FSH] level <10 mIU/mL, luteinizing hormone [LH] level <10 mIU/mL) and regular menstrual cycles. The patients had no history of prior chemotherapy or hormone therapy. The institutional review board at Samsung Medical Center, Sungkyunkwan University School of Medicine, approved this study protocol.
Before initiation of chemotherapy, a hormonal profile of FSH, LH, estradiol, progesterone, prolactin, and TSH levels was obtained and a subcutaneous injection of leuprolide acetate (3.75 mg, Leuplin, Takeda Chemical Industries Ltd., Osaka, Japan) was given. To avoid possible toxic effects of the chemotherapeutic agents prior to ovarian suppression, chemotherapy was not started until ovarian suppression was confirmed by measurement of serum estradiol level (<30 pg/mL), taken 2 weeks after leuprolide acetate treatment. The chemotherapy regimens were determined by oncologists according to individual cancer characteristics and patient prognosis and the patients with estrogen receptor positive tumors were provided tamoxifen medication from 3 weeks after the completion of chemotherapy. Thereafter, leuprolide acetate was administered every 4 weeks until the completion of chemotherapy. The cut-off values of increased FSH and LH were >20 mIU/mL for each hormone. In addition, daily calcium supplementation (1,500 mg/d) was recommended.
Nine patients received 600 mg/m2 cyclophosphamide and 60 mg/m2 doxorubicin every 3 weeks for four cycles. Seven patients received 600 mg/m2 cyclophosphamide, 60 mg/m2 doxorubicin, and 175 mg/m2 paclitaxel every 3 weeks for four cycles. Six patients received 500 mg/m2 cyclophosphamide, 50 mg/m2 doxorubicin, and 500 mg/m2 5-fluorouracil every 3 weeks for six cycles.
Follow-up laboratory tests to measure LH, FSH, estradiol, and progesterone were performed 1, 3, and 6 months after completion of chemotherapy. For statistical analysis, independent t-test or Fisher's exact test, Wilcoxon Signed Ranks test, and Kruskal-Wallis test with LSD were used. A P value <0.05 was considered significant. Menstrual patterns and symptoms of ovarian failure were observed for a mean duration of 35.6±1.79 months (mean±SD). The patients were questioned about their menstruation and menopausal symptoms (hot flashes, sweating, and vaginal dryness). The telephone interview was performed in those patients who did not visit the hospital after the last serum sample was collected.
As a preliminary study, we used GnRHa in all patients because ethically all patients should receive the potentially protective treatment. Although the current study had no control group, the assessment of serial hormonal levels should reflect the effects of GnRHa treatment. Therefore, instead of recruiting control patients, our results were compared with those already described in the medical literature for similar patients who received chemotherapy without GnRHa.
RESULTS
Demographic and clinical profiles of breast cancer patients
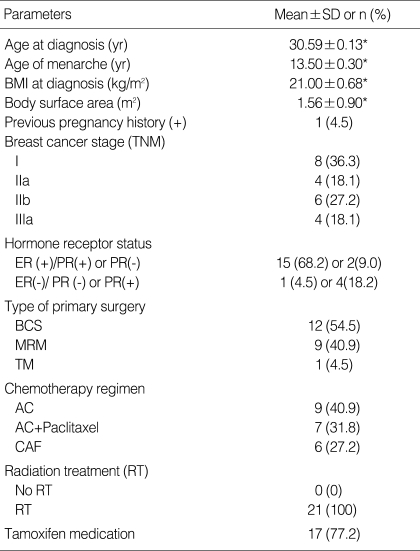
Patient characteristics are summarized in Table 1. The mean age at diagnosis was 30.6±5.1 yr, and the mean age of menarche was 13.5±6.3 yr. All patients had regular menstrual cycles prior to chemotherapy and 4.5% (1/22) of the patients had been pregnant previously. Prior to chemotherapy, the mean body mass index (BMI) was 21.0±0.7 kg/m2 and the mean body surface area was 1.6±0.9 m2. Eight women had stage I or II disease and four women had stage III disease. Breast conservation surgery was performed in 54.5% (12/22) of patients. Patients with estrogen receptor-positive tumors received tamoxifen (17/22). All patients received radiation therapy that excluded the pelvis.
Table 1.
Demographic and clinical profiles of breast cancer patients
*Data expressed as mean±SD.
BCS, Breast conservative surgery; MRM, Modified radical mastectomy; TM, Total mastectomy; AC, doxorubicin, cyclophosphamide; CAF, cyclophosphamide, doxorubicin, 5-Fluorouracil.
Chemotherapeutic agents
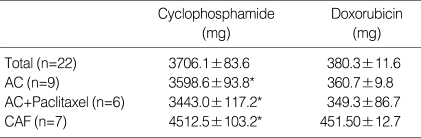
Nine patients (41%) received four courses of doxorubicin and cyclophosphamide (AC) with four courses of paclitaxel, seven patients (31.8%) received four courses of AC only, and six patients (27.2%) received six cycles of the cyclophosphamide, doxorubicin, and 5-fluorouracil (CAF) regimen. The total doses of cyclophosphamide and of doxorubicin were 3741.3±15.7 mg and 381.3±12.4 mg, respectively. The total dose of cyclophosphamide was significantly higher in the CAF regimen than in the AC or AC+paclitaxel regimen (4,512.5±103.2 vs. 3,598.6±93.8 or 3,443.0±117.2, respectively; P<0.05) (Table 2).
Table 2.
Total doses of chemotherapeutic agents
Data are expressed as mean±SD.
*P<.05 by Wilcoxon Signed Ranks test.
40.9% of patients (9/22) received four cycles of cyclophosphamide and doxorubicin. 31.8% of patients (7/22) received four cycles of cyclophosphamide, doxorubicin, and paclitaxel. 27.2% of patients (6/22) received six courses of CAF (cyclophosphamide, doxorubicin, 5-fluorouracil). Total doses of cycloph-osphamide were significantly higher in the CAF regimen than in the AC or AC+P regimens (P<.05).
AC, doxorubicin, cyclophosphamide; CAF, cyclophosphamide doxorubicin, 5-Fluorouracil.
Gonadotropin levels
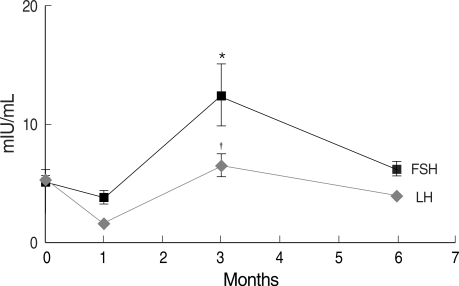
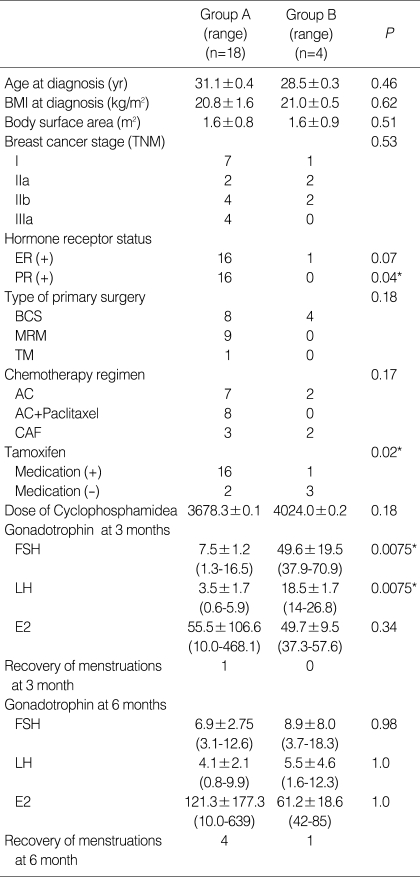
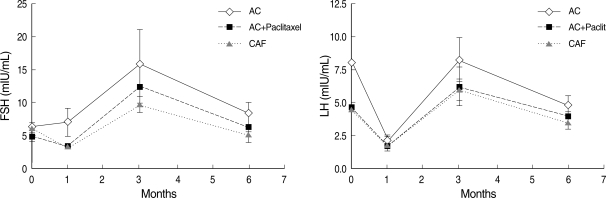
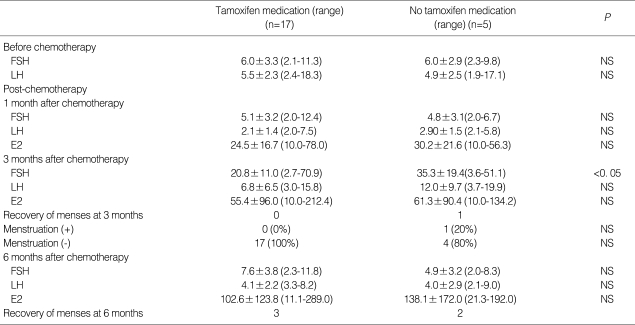
Gonadotropin levels were evaluated at 1, 3, and 6 months after the completion of chemotherapy. The mean time between the last GnRHa injection and the first sampling was 44.0±19.2 (mean±SD, range: 28-55) days. Mean serum FSH and LH levels were similar to baseline 6 months after completion of chemotherapy (8.0±5.3 mIU/mL and 4.4±2.7 mIU/mL, respectively); mean serum estradiol was 110.4±l61.5 pg/mL. Overall, compared to baseline, gonadotropin levels were significantly different 3 months after completion of chemotherapy, while levels were in the normal range at 1 and 6 months (Fig. 1). In four patients, serum FSH 3 months after completion was significantly higher than at 1 and 6 months (49.6±19.5 IU/mL) (P<0.05) (Table 3) and each values of gonadotropin (FSH/LH) in these patients were 38.4/14.0, 51.1/17.0, 37.9/16.2 and 70.9/26.8 mIU/mL. However, despite this transient elevation, gonadotropin levels were restored to the normal range 6 months after chemotherapy. There were no significant differences between the two groups except for the use of tamoxifen medication and a progesterone receptor positive status (P<0.05) (Table 3). The total dose of cyclophosphamide was the same between patients with elevated FSH and patients with normal levels. In addition, there were no statistically significant differences in gonadotropin levels after completion of chemotherapy when different chemotherapy regimens were compared (Fig. 2). The gonadotropin levels were normal 6 months after the completion of chemotherapy regardless of tamoxifen medication (Table 4). In the patients with using tamoxifen, one showed elevated FSH (51.1 mIU/mL) and her period was recovered at 13 months after the completion of chemotherapy.
Fig. 1.
Overall gonadotropin levels before and after completion of chemotherapy.
*Serum FSH levels 3 months after completion of chemotherapy were significantly higher than those at 1 month or 6 months (P<0.05); †Serum LH levels 3 months after completion of chemotherapy were also significantly higher than those at 1 month or 6 months (P<0.05).
*,†P<0.05 is considered significant by Kruskal-Wallis test with LSD.
Table 3.
Clinical profiles of patients with no increase in FSH or LH 3 months after completion of chemotherapy (Group A) and patients with increased FSH and LH 3 months after completion of chemotherapy (Group B)
Data are expressed as mean±SD.
P values by independent t-test or Fisher's exact test.
Group A, Patients with no increase in FSH or LH 3 months after completion of chemotherapy; Group B, Patients with a significant increase in FSH and LH 3 months after completion of chemotherapy.
Each values of gonadotropin (FSH/LH) were 38.4/14.0, 51.1/17.0, 37.9/16.2 and 70.9/26.8 mIU/mL in Group B.
FSH, follicle stimulating hormone; BMI, body mass index; BCS, breast consening surgery; MRM, modified radical mastectomy; TM, total mastectomy; AC, doxorubicin, cyclophosphamide; CAF, cyclophosphamide doxorubicin, 5-Fluorouracil.
Fig. 2.
Gonadotropin levels according to the chemotherapy regimen.
There was no significant correlation between serum gonadotropin levels and type of chemotherapy regimen (P>0.05).
P values by Kruskal-Wallis test with LSD.
AC, doxorubicin, cyclophosphamide; CAF, cyclophosphamide doxorubicin, 5-Fluorouracil.
Table 4.
Gonadotropin levels in patients with and without tamoxifen treatment after chemotherapy
Data are expressed as mean±SD.
P values by independent t-test or Fisher's exact test.
Symptoms of ovarian failure
During the 6 months of follow-up, none of the patients exhibited menopausal symptoms. Among those patients with normal FSH levels at the 3-month follow-up, 61.1% (15/18) experienced recovery of menstruation within 6 months, while 75% of patients (3/4) with elevated FSH at the 3-month follow-up experienced menstrual cycle recovery. Symptom follow-up of a mean duration of 35.6±1.7 months post-chemotherapy showed that 18 patients (81.8%) experienced menstrual cycle recovery. One patient became pregnant within a year of treatment completion and gave birth to a normal infant; another woman became pregnant, but the pregnancy was terminated.
DISCUSSION
Advances in adjuvant chemotherapy for breast cancer have led to improved long-term survival; however, chemotherapeutic agents may cause profound long-term sequelae, including damage to ovarian function in young patients (7-9). Ovarian function is essential not only because it preserves fertility in cancer survivors, but also because it prevents the risk of premature ovarian failure and the morbidities associated with a hypoestrogenic state, including vasomotor symptoms, osteoporosis, urogenital symptoms, and heart disease (3, 10, 11). It has been reported that approximately 64% of adult women undergoing cancer therapy experience one or more symptoms of ovarian failure (3).
Several approaches have been taken to preserve ovarian function or fertility during chemotherapy including oophoropexy, ovarian suppression, and cryopreservation of ovarian tissue, oocytes, and embryos. None of these options have proven efficacy except embryo cryopreservation, which has its own limitations of requiring a current suitable sperm donor and a surgical procedure. In contrast to these procedures, GnRHa injections are easily performed without surgery. Because dividing cells are known to be more sensitive to cytotoxic drugs than cells at rest, it has been suggested that inhibition of the pituitary-gonadal axis could reduce the rate of oogenesis and thereby render the germinal epithelium less susceptible to cytotoxicity. Therefore, GnRHa, acting at an earlier stage of follicular development, might prevent follicles from reaching the chemotherapy-sensitive stage (4). An argument against the efficacy of GnRHa is that, theoretically, GnRH analogues preserve only follicles that have initiated growth, which constitute <10% of the entire follicular pool in the ovary at any given time. The ovarian reserve comprises 90% primordial follicles that initiate follicle growth through an unknown FSH-independent mechanism. In addition, primordial follicles do not express FSH or GnRH receptors, which are uniformly present as early as the third or fourth granulosa layer of preantral follicles. Nevertheless, current biological explanations are not sufficient to explain the effects of GnRHa on ovarian reserve.
Despite its unknown mechanism, clinical studies have shown that ovarian suppression by GnRHa can have a protective effect on ovarian function during chemotherapy. However, information regarding the effectiveness of this treatment in breast cancer patients is limited, especially among women of reproductive age, as most prior studies have evaluated women with leukemia or Hodgkin's lymphoma. Ataya et al. (12) showed in rhesus monkeys that administration of GnRHa in parallel with cyclophosphamide decreased the daily rate of follicular decline and the total number of follicles lost significantly, compared with cyclophosphamide alone. In addition, Blumenfeld et al. (13) reported that 96% of patients in their study who received both chemotherapy and GnRHa resumed spontaneous ovulatory cycles, while only 35% of patients receiving chemotherapy alone did. In a study by Recchia et al. in young women with breast cancer (5), all patients who received GnRHa and adjuvant chemotherapy resumed normal menses. The study enrolled 100 patients and the mean follow-up duration was 75 months. Although the total number of patients in our current study is smaller and the follow-up duration shorter, the median patient age is much younger than in the study by Recchia et al. (31 yr vs. 43 yr, respectively). Our study is unique in that it focuses on breast cancer patients in their critical period of fertility (younger than the mid-30s). By limiting enrollment to women of reproductive age, this study evaluated the efficacy of GnRHa on protecting ovarian function during adjuvant chemotherapy in women whose lives would be most affected by ovarian damage.
We administered GnRHa 2 weeks prior to the initiation of chemotherapy and confirmed ovarian suppression by serum estradiol levels. A delay between GnRHa administration and chemotherapy initiation is thought to provide better ovarian protection than concurrent administration. Serial measurement of gonadotropin levels demonstrated that they were normal 6 months after chemotherapy. At the 6-month follow-up, 63.6% of patients experienced regular menstruation. Patients were followed for a mean duration of 35.6±1.7 months and 81.8% (18/22) experienced recovery of menstruation. The patients who did not resume menstruation (n=4) were all taking tamoxifen during follow-up.
The mean total dose of cyclophosphamide in this study was 3,706.10±83.60 mg, although there were significant differences between regimens (CAF vs. AC vs. AC+paclitaxel: 4,512.5±103.2 vs. 3,598.6±93.8 vs. 3,443.0±117.2, respectively; P<0.05). The gonadotoxic dose of cyclophosphamide (single-drug regimen) is reported to be 5,200 mg, 9,300 mg, and 20,400 mg among women in their 40s, 30s, and 20s, respectively (14). Although each dose of cyclophosphamide in our study was lower than the cumulative gonadotoxic dose before the onset of amenorrhea, gonadotoxicity occurs at a lower dose when cyclophosphamide is used in combination chemotherapy than with a single-drug regimen (15-17). For example, the American Society of Clinical Oncology reported that the risk of permanent amenorrhea among women in their 30s receiving CAF, where the total dose of cyclophosphamide is also lower than in the single drug regimen, is intermediate (20-80%) (18). Because of synergistic toxicity, the gonadotoxic effects of combination chemotherapy cannot be interpreted based on the dosage of the chemoagent alone.
At the 3-month follow-up, decreased ovarian reserve (FSH >20 mIU/mL) was suspected in four patients. Their FSH levels had returned to normal by the 6-month follow-up, and three had resumed their normal menstrual cycles. The characteristics of these four patients were compared with those without an elevated FSH at the 3-month follow-up (Table 3). The rate of decreased ovarian reserve was higher in patients who did not receive tamoxifen (P<0.05). On this finding, it could suggest that tamoxifen prevent the transient elevation of hormone level. But, it needs more studies to conclude that tamoxifen suppresses the elevation of gonadotropin level, because the number of patients is small and the level of FSH at 6 months did not show difference. A similar finding of elevated FSH at the 3-month follow-up after completing chemotherapy was reported in a study by Blumenfeld (19) on monthly GnRHa injections in 44 women with lymphoma receiving chemotherapy, aged 15 to 40 yr. Temporarily increased FSH concentrations were detected in nearly one-third of the patients who resumed normal ovarian function. The mechanism of this phenomenon is unknown, but this finding suggests that the gonadotropin level should be monitored for at least 6 months after completing chemotherapy to thoroughly evaluate ovarian function. These preexisting markers could not estimate ovarian reserve quantitatively and, in the future, AMH, regarded as a novel marker of ovarian reserve, may be useful for evaluating serial change of ovarian reserve.
Despite the promising results of our study, we cannot conclude that ovarian suppression by GnRHa effectively preserves ovarian function in young breast cancer patients receiving adjuvant chemotherapy due to the lack of a control group. Fortunately, several prospective randomized trials are presently ongoing: the Zoladex Rescue of Ovarian Function (ZORO) study in Germany, a multicenter study of Italian breast cancer patients, and a study by the Southwestern Oncology Group (SWOG-S0230). If these trials demonstrate a beneficial effect adotoxicity, clinical data on GnRHa treatment in young breast cancer patients in Korea will need to be obtained in the near future. Until then, our study will hopefully help clinicians to present more informed options to young breast cancer patients seeking methods to preserve their fertility.
References
- 1.Jatoi I, Miller AB. Why is breast-cancer mortality declining? Lancet Oncol. 2003;4:251–254. doi: 10.1016/s1470-2045(03)01037-4. [DOI] [PubMed] [Google Scholar]
- 2.Ataya K, Moghissi K. Chemotherapy-induced premature ovarian failure: mechanisms and prevention. Steroids. 1989;54:607–626. doi: 10.1016/0039-128x(89)90084-6. [DOI] [PubMed] [Google Scholar]
- 3.Blumenfeld Z, Avivi I, Linn S, Epelbaum R, Ben-Shahar M, Haim N. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum Reprod. 1996;11:1620–1626. doi: 10.1093/oxfordjournals.humrep.a019457. [DOI] [PubMed] [Google Scholar]
- 4.Pereyra Pacheco B, Mendez Ribas JM, Milone G, Fernandez I, Kvicala R, Mila T, Di Noto A, Contreras Ortiz O, Pavlovsky S. Use of GnRH analogs for functional protection of the ovary and preservation of fertility during cancer treatment in adolescents: a preliminary report. Gynecol Oncol. 2001;81:391–397. doi: 10.1006/gyno.2001.6181. [DOI] [PubMed] [Google Scholar]
- 5.Recchia F, Saggio G, Amiconi G, Di Blasio A, Cesta A, Candeloro G, Rea S. Gonadotropin-releasing hormone analogues added to adjuvant chemotherapy protect ovarian function and improve clinical outcomes in young women with early breast carcinoma. Cancer. 2006;106:514–523. doi: 10.1002/cncr.21646. [DOI] [PubMed] [Google Scholar]
- 6.Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embryo, oocytes or ovaries. Oncologist. 2007;12:1044–1054. doi: 10.1634/theoncologist.12-9-1044. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17:2365–2370. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- 8.Chapman RM, Sutcliffe SB, Malpas JS. Cytotoxic-induced ovarian failure in women with Hodgkins disease. I. Hormone function. JAMA. 1979;242:1877–1881. [PubMed] [Google Scholar]
- 9.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 10.Santoro A, Bonadonna G, Valagussa P, Zucali R, Viviani S, Villani F, Pagnoni AM, Bonfante V, Musumeci R, Crippa F. Long-term results of combined chemotherapy-radiotherapy approach in Hodgkin's disease: superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. J Clin Oncol. 1987;5:27–37. doi: 10.1200/JCO.1987.5.1.27. [DOI] [PubMed] [Google Scholar]
- 11.Bokemeyer C, Schmoll HJ, van Rhee J, Kuczyk M, Schuppert F, Poliwoda H. Long-term gonadal toxicity after therapy for Hodgkin's and non-Hodgkin's lymphoma. Ann Hematol. 1994;68:105–110. doi: 10.1007/BF01727413. [DOI] [PubMed] [Google Scholar]
- 12.Ataya K, Rao LV, Lawrence E, Kimmel R. Luteinizing hormonereleasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod. 1995;52:365–372. doi: 10.1095/biolreprod52.2.365. [DOI] [PubMed] [Google Scholar]
- 13.Blumenfeld Z, Avivi I, Ritter M, Rowe JM. Preservation of fertility and ovarian function and minimizing chemotherapy-induced gonadotoxicity in young women. J Soc Gynecol Investig. 1999;6:229–239. doi: 10.1016/s1071-5576(99)00028-3. [DOI] [PubMed] [Google Scholar]
- 14.Koyama H, Wada T, Nishizawa Y, Iwanaga T, Aoki Y. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer. 1997;39:1403–1409. doi: 10.1002/1097-0142(197704)39:4<1403::aid-cncr2820390408>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Nabholtz J, Peinkowski T, Mackey J. Phase III trial comparing TAC with RAC in the adjuvant treatment of node positive breast cancer patients:interim analysis of the BCIRG001 study. Pro Am Soc Clin Oncol. 2002;21:36a. [Google Scholar]
- 16.Kil WJ, Ahn SD, Shin SS, Lee WS, Choi EK, Kim JH, Son BH, Ahn SH, Kim WK, Kim SB. Treatment-induced menstrual changes in very young (<35 years old) breast cancer patients. Breast Cancer Res Treat. 2006;96:245–250. doi: 10.1007/s10549-005-9059-x. [DOI] [PubMed] [Google Scholar]
- 17.Arriagada R, Le MG, Spielmann M, Mauriac L, Bonneterre J, Namer M, Delozier T, Hill C, Tursz T. Randomized trial of adjuvant ovarian suppression in 926 premenopausal patients with early breast cancer treated with adjuvant chemotherapy. Ann Oncol. 2005;16:389–396. doi: 10.1093/annonc/mdi085. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 19.Blumenfeld Z. Ovarian rescue/protection from chemotherapeutic agents. J Soc Gynecol Investig. 2001;8:S60–S64. doi: 10.1016/s1071-5576(00)00112-x. [DOI] [PubMed] [Google Scholar]