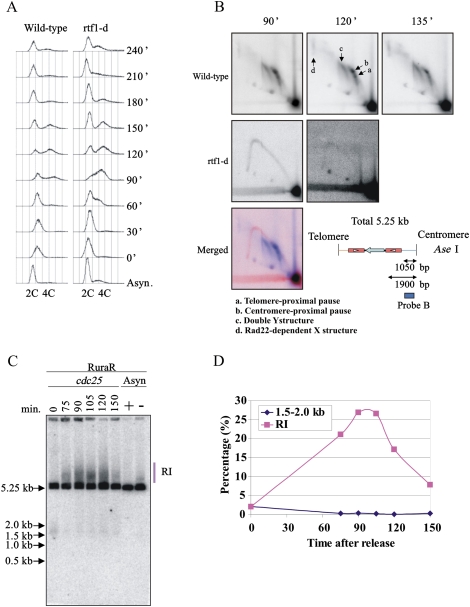
Figure 6.
Absence of a detectable DSB induction in synchronized cells. RuraR rtf1+ and RuraR rtf1-d cells were grown without thiamine (14 h) and were synchronized by cdc25.22 block and release. The time course starts after cell cycle release. Asynchronous cultures were grown for 24 h with (−; pause off) and without (+; pause on) thiamine as a control where appropriate. (A) FACS analysis. The culture before the cell cycle arrest was used as asynchronous control culture “Async.” S phase occurs between 60 and 150 min. (B) Two-dimensional gel electrophoresis at specified time points with (rtf1+) and without (rtf1-d) fork arrest (a–d as indicated) (see Supplemental Fig. S1). (Bottom) Map of AseI digest for RuraR. The position of the Cen probe is indicated by the bar. In the merged picture, the signal corresponding to 90 min for rtf1-d is red and that for rtf1+ is blue. (C) Southern hybridization of one-dimensional gel to visualize replication intermediates (RI) from the indicated times. DNA was digested with AseI. (D) Quantification of one-dimensional gel showing the percentage total signal present in replication intermediates (red squares) and at the position of a predicted break (blue diamonds). Forks broken within the Cen-proximal RTS1 would generate a band of ∼1500 bp. A faint signal between 1.5 and 2.0 kb is seen in the G2-arrested sample (0 min), but this is lost when cells enter S phase (75 min).