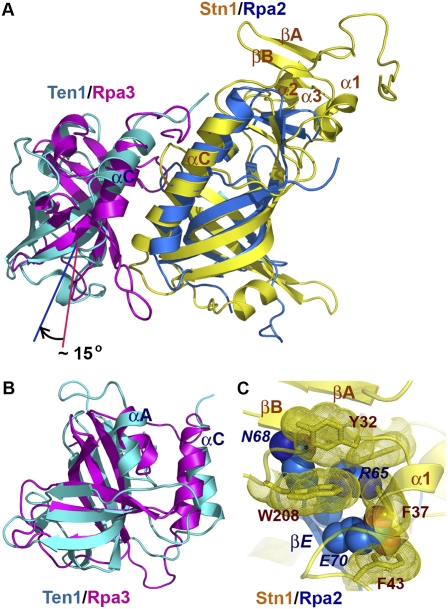
Figure 4.
The C. tropicalis Stn1N–Ten1 complex is structurally similar to Rpa2N–Rpa3. (A) Superposition of the Stn1N–Ten1 complex on the crystal structure of the human Rpa2N–Rpa3 complex (Bochkarev et al. 1999). Stn1N and Ten1 are colored in yellow and cyan and Rpa2N and Rpa3 are shown in blue and magenta. The superposition is based on the structures of Stn1N and Rpa2N. Ten1 and Rpa3 are not aligned well, and Ten1 rotates ∼15° relative to the orientation of Rpa3. (B) Overlay of Ten1 and Rpa3 based on the OB fold β barrels of the proteins. (C) Superposition of Stn1N and Rpa2N based on the OB fold β barrels shows collisions between the cap motif of Stn1N and the N-terminal β hairpin (βD–βE) of Rpa2N. Residues in Stn1N are drawn as a stick model with dotted surface. Residues in Rpa2N are shown as a space-filling model. Stn1N and Rpa2N are colored as in A. Labels for residues in Rpa2 are in italics, to differentiate them from residues in Stn1.