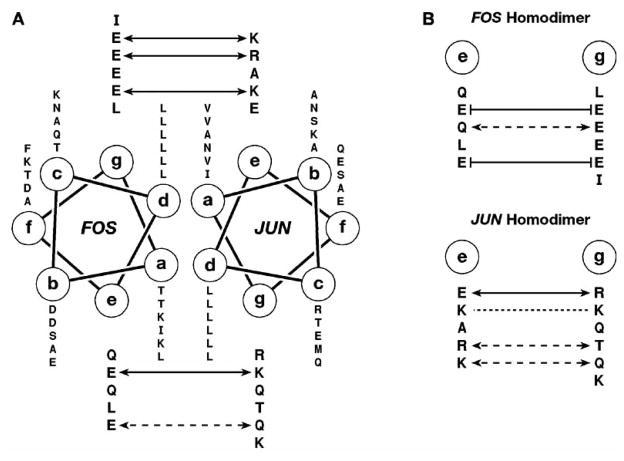
Fig. 4.
Mechanism of dimerization specificity for Fos and Jun peptides. (A) A helical wheel diagram of the Fos–Jun coiled-coil. The sequence is read from N to C termini outwards from the wheel. The Fos–Jun heterodimer is stabilized by four electrostatic attractive interactions (solid arrows) and by a hydrogen bond interaction (dashed arrows) between the g and e positions. (B) Potential interactions within homodimer interfaces of Fos and Jun. The formation of Fos homodimer is prevented by four electrostatic repulsive interactions (solid lines). The formation of Jun homodimer is driven by two positive electrostatic interactions and it is additionally stabilized by hydrogen bonds and the interactions between Lys residues (dotted line), wherein the repulsive electrostatic energies between like charges are overcome by the favorable Van der Waals interactions between the methylenes of lysine side chains and hydrophobic amino acids in the a and d positions [85].