Figure 5.
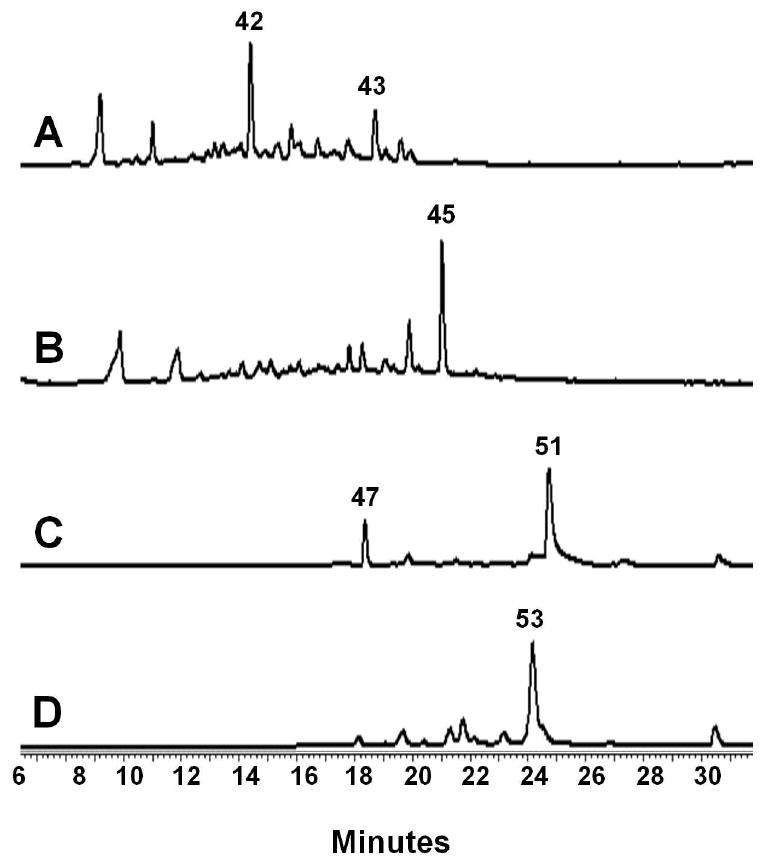
Reconstitution of tetracycline intermediates using ssf genes expressed in S. lividans K4-114. (A) HPLC analysis (245 nm) of the K4-114/pLP27 extract shows the amidated, reduced polyketide 42 is the major product, confirming the biosynthesis of the polyketide backbone 40 by SsfABCD and the subsequent C-9* reduction by SsfU. (B) HPLC analysis (253 nm) of the K4-114/pLP27/pLP77 extract shows addition of the putative cyclase SsfY1 leads to complete cyclization and aromatization of the D and formation of the shunt benzopyrone 44. (C) HPLC analysis (430 nm) of K4-114/pLP27/pLP126 extract shows 50, the oxidized form of 49, as the dominant product. Biosynthesis of 49 using entirely ssf genes (SsfABCDUY1Y2M4L2) indicates the tetracycline nature of the ssf biosynthetic pathway. (D) HPLC analysis (395 nm) of K4-114/pLP36 extract confirms the function of SsfO2 as the oxygenase that dihydroxylated C-4 and C-12a of 49. The resulting product 51 undergoes spontaneous degradation to afford the observed product 52.
