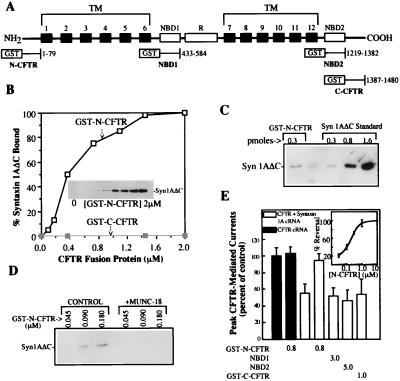
Figure 1.
Syntaxin 1A directly binds to the N-terminal cytoplasmic tail of CFTR. (A) Schematic view of the major CFTR domains and the locations of the GST fusion proteins used in this study. TM, transmembrane. (B) Binding of syntaxin 1A cytosolic domain (0.35 nmol) to increasing concentrations of GST-N-CFTR (Inset and □) in a 0.2-ml reaction volume. No syntaxin 1A binding to GST-C-CFTR was observed (■). Results are representative of four different experiments. (C) syn1AΔC binds to GST-N-CFTR with an approximately 1:1 stoichiometry. syn1AΔC (350 pmol) was mixed with 0.3 pmol of GST-N-CFTR, and the amount of syn1AΔC that bound was estimated by immunoblotting using known amounts of syn1AΔC as standards. (D) Munc-18a blocks the binding of GST-N-CFTR to syn1AΔC. GST-N-CFTR at the indicated concentrations was mixed with 0.1 μg of syn1AΔC in the absence or presence of 0.2 μg of Munc-18a in a 0.2-ml reaction volume. (E) GST-N-CFTR peptide blocks the inhibition of CFTR currents by membrane-anchored syntaxin 1A. Oocytes (10–12 oocytes per condition) that were expressing CFTR with or without full-length syntaxin 1A were microinjected with the indicated GST-CFTR fusion proteins (units: μM) 30 min prior to current recording (see text and ref. 6 for details). Error bars represent SEMs. The dose dependence of the block of the CFTR–syntaxin 1A interaction by GST-N-CFTR is shown in the Inset (five oocytes per concentration).